Publication 2006


▼Enantioselective Diels-Alder Reaction of a-Acyloxyacroleins Catalyzed by Chiral 1,1'-Binaphthyl-2,2'-diammonium Salts
Akira Sakakura
Kenji Suzuki
Kazuaki Ishihara*
Adv. Synth. Catal. 2006, 348, 2457-2465.
The use of BINAP-based palladium(0) catalysts in the reaction of 7-methoxy-1,2-didehydronaphthalene (2) and dimethyl acetylenedicarboxylate (DMAD) affords non-racemic 9,12-dimethoxypentahelicene 1 with reasonable ees, among other cycloaddition products. This is the first example of an enantioselective, metal-catalyzed cycloaddition involving arynes.
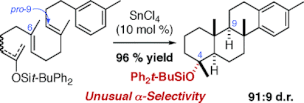
▼Catalytic Diastereoselective Polycyclization of Homo(polyprenyl)arene Analogues Bearing Terminal Siloxyvinyl Groups
Muhammet Uyanik
Kazuaki Ishihara*
Hisashi Yamamoto*
Org. Lett. 2006, 8, 5649-5652.
Highly diastereoselective polycyclization of homo(polyprenyl)arene analogues bearing terminal siloxyvinyl groups was catalyzed by tin(IV) chloride (10 mol %). The cyclizations of tert-butyldiphenylsilyl and triisopropylsilyl polyenol ethers gave 4(equatorial)- and 4(axial)-siloxypolycycles as major isomers, respectively. The strong nucleophilicity of pro-C(9), a (6E) geometry, and a bulky silyl group effectively favored the 4-preference, whereas the weak nucleophilicity of pro-C(9), a (6Z)-geometry, and less steric hindrance of a silyl group favored the 4-preference.

▼Dimeric Scandium(III) and Monomeric Lanthanide(III) Complexes with Perfluoropropane-1,3-disulfonates as Counter Anions for Lewis Acid Catalysis
Manabu Hatano
Eri Takagi
Manabu Arinobe
Kazuaki Ishihara*
J. Organometal. Chem. 2007, 692, 569-578.
▷http://dx.doi.org/10.1016/j.jorganchem.2006.05.057
A novel scandium(III) complex with disulfonates as counter anions, [Sc(μ-OH)(H2O)5]2[O3S(CF2)3SO3]2 (5), was prepared from scandium oxides (Sc2O3) and perfluoropropane-1,3-disulfonic acid (1, HO3SCF2CF2CF2SO3H). By X-ray analysis, 5 was found to be a μ-OH-bridged dimeric structure bearing two perfluoropropane-1,3-disulfonates without bonding to scandium(III) centers. A series of lanthanide(III) complexes were also prepared from 1 and lanthanide oxides (Ln2O3; Ln = La, Nd, Sm, and Gd). In sharp contrast to the dimeric scandium(III) complex, the corresponding lanthanide(III) complexes had monomeric structures. Interestingly, the dimeric scandium(III) complex, but not the monomeric lanthanide complexes, with perfluoropropane-1,3-disulfonates served as an efficient Lewis acid catalyst for the hydrolysis of esters.

▼Iron(III)-Zirconium(IV) Combined Salt Immobilized on N-(Polystyrylbutyl)pyridinium Triflylimide as a Reusable Catalyst for a Dehydrative Esterification Reaction
Yuka Nakamura
Toshikatsu Maki
Xiaowei Wang
Kazuaki Ishihara*
Hisashi Yamamoto*
Adv. Synth. Catal. 2006, 348, 1505-1510.
N-(Polystyrylbutyl)pyridinium triflylimide is prepared by a coupling reaction of commercially available 4-bromobutyl-polystyrene with pyridine and a subsequent anion exchange reaction with lithium triflylimide. This new polystyrene-bound pyridinium salt is useful as a solid support to immobilize a zirconium(IV)-iron(III) binary metal complex, which is a homogeneous catalyst for the dehydrative ester condensation of an equimolar mixture of carboxylic acids and alcohols. The immobilized zirconium(IV)-iron(III) complex is easily recovered by filtration after complete esterification, and is reusable without any loss of activity.

▼Highly Efficient Alkylation to Ketones and Aldimines with Grignard Reagents Catalyzed by Zinc(II) Chloride
Manabu Hatano
Shinji Suzuki
Kazuaki Ishihara*
J. Am. Chem. Soc. 2006, 128(31), 9998-9999.
A highly efficient alkylation to ketones and aldimines with Grignard reagents in the presence of catalytic trialkylzinc(II) ate complexes derived from ZnCl2 (10 mol%) in situ was developed. This simple Zn(II)-catalyzed alkylation could minimize the well known but serious problems with the use of only Grignard reagents which lead to reduction and aldol side products, and the yield of desired alkylation products could be improved.

▼3,3’-Diphosphoryl-1,1’-bi-2-naphthol-Zn(II) Complexes as Conjugate Acid-Base Catalysts for Enantioselective Dialkylzinc Addition to Aldehydes
Manabu Hatano
Takashi Miyamoto
Kazuaki Ishihara*
J. Org. Chem. 2006, 71(17), 6474-6484.
A highly enantioselective dialkylzinc (R22Zn) addition to a series of aromatic, aliphatic, and heteroaromatic aldehydes (5) was developed based on conjugate Lewis acid?Lewis base catalysis. Bifunctional BINOL ligands bearing phosphine oxides [P(=O)R2] (7), phosphonates [P(=O)(OR)2] (8 and 9), or phosphoramides [P(=O)(NR2)2] (10) at the 3,3’-positions were prepared by using a phospho-Fries rearrangement as a key step. The coordination of a NaphO-Zn(II)-R2 center as a Lewis acid to a carbonyl group in a substrate and the activation of R22Zn(II) with a phosphoryl group (P=O) as a Lewis base in the 3,3’-diphosphoryl-BINOL-Zn(II) catalyst could promote carbon-carbon bond formation with high enantioselectivities (up to >99% ee). Mechanistic studies were performed by X-ray analyses of a free ligand (7) and tetranuclear Zn(II) cluster (21), a 31P NMR experiment on Zn(II) complexes, an absence of non-linear effect between the ligand (7) and Et-adduct of benzaldehyde, and stoichiometric reactions with some chiral or achiral Zn(II) complexes, to propose a transition-state assembly including monomeric active intermediates.

▼Enantioselective Dialkylzinc Addition to Aldehydes Catalyzed by Chiral Zn(II)-BINOLates Bearing Phosphonates and Phosphoramides in the 3,3’-Positions
Manabu Hatano
Takashi Miyamoto
Kazuaki Ishihara*
Synlett 2006, 1762-1764.
Highly enantioselective dialkylzinc addition to a series of aldehydes was developed based on chiral Zn(II)-BINOLate catalysts bearing phosphonates [P(=O)(OR)2] and phosphoramides [P(=O)(NMe2)2] at the 3,3'-positions. The reactions proceeded smoothly and showed reductions in the amounts of both catalysts and dialkylzinc reagents to be loaded.

▼Design of Brnsted Acid-Assisted Chiral Brensted Acid Catalyst Bearing a Bis(triflyl)methyl Group for a Mannich-Type Reaction
Aiko Hasegawa
Yuki Naganawa
Makoto Fushimi
Kazuaki Ishihara*
Hisashi Yamamoto*
Org. Lett. 2006, 8(15), 3175-3178.
A new Brnsted acid-assisted chiral Brnsted (chiral BBA) acid catalyst (1) was developed by substituting a hydroxy group of optically active 1,1'-bi(2-naphthol) with a stronger Brønsted acidic group such as a bis(trifluoromethanesulfonyl)methyl group. The enantioselective Mannich-type reaction of ketene silyl acetals with aldimines catalyzed by (R)-1 in the presence of stoichiometric achiral proton sources gave (S)--amino esters in high yield with moderate to good enantiomeric excesses.
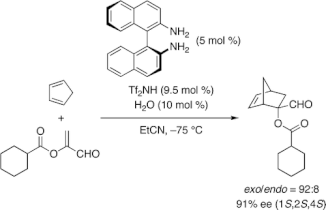
▼Chiral 1,1‘-Binaphthyl-2,2‘-diammonium Salt Catalysts for the Enantioselective Diels−Alder Reaction with α-Acyloxyacroleins
Akira Sakakura
Kenji Suzuki
Kazuhiko Nakano
Kazuaki Ishihara*
Org. Lett. 2006, 8(11), 2229-2232.
▷DOI: 10.1021/ol060490l
A diammonium salt of chiral 1,1‘-binaphthyl-2,2‘-diamine and trifluoromethanesulfonimide (Tf2NH) shows excellent catalytic activity and enantioselectivity for the Diels−Alder reaction of α-acyloxyacroleins with cyclic dienes. For example, in the presence of 5 mol % of the ammonium catalyst, the Diels−Alder reaction of α-(cyclohexanecarbonyloxy)acrolein with cyclopentadiene proceeded in EtCN at −75 °C to give the adducts in 88% yield with 92% exo and 91% ee. This catalyst can be easily prepared in situ by mixing the commercially available chiral diamine and Tf2NH.

▼Design of a Small-Molecule Catalyst Using Intramolecular Cation- Interactions for Enantioselective Diels-Alder and Mukaiyama-Michael Reactions: L-DOPA-Derived Monopeptide-Cu(II) Complex
Kazuaki Ishihara*
Makoto Fushimi
Org. Lett. 2006, 8, 1921-1924.
We have designed a small-molecule artificial metalloenzyme that is prepared in situ from Cu(OTf)2 or Cu(NTf2)2 (1.0 equiv) and L-DOPA-derived monopeptide (1.1 equiv). This catalyst (2-10 mol %) is highly effective for the enantioselective Diels-Alder (DA) and Mukaiyama-Michael (MM) reactions with ,-unsaturated 1-acyl-3,5-dimethylpyrazoles. The present results demonstrate that cation- interactions may be available for controlling the conformation of sidearms of chiral ligands, and monopeptides are readily tunable ligands that include only one chiral center.
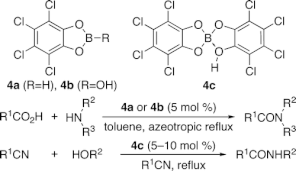
▼4,5,6,7-Tetrachlorobenzo[d][1,3,2]dioxaborol- 2-ol as an Effective Catalyst for the Amide Condensation of Sterically Demanding Carboxylic Acids
Toshikatsu Maki
Kazuaki Ishihara*
Hisashi Yamamoto*
Org. Lett. 2006, 8, 1431-1434.
▷DOI:
10.1021/ol060216r
4,5,6,7-Tetrachlorobenzo[d][1,3,2]dioxaborole (4a) and 4,5,6,7-tetrachlorobenzo[d][1,3,2]dioxaborol-2-ol (4b) are effective catalysts for the dehydrative amide condensation between an equimolar mixture of carboxylic acids and amines. In particular, these catalysts are greatly superior to 3,5-bis(trifluoromethyl)phenylboronic acid (1) for the amide condensation of sterically demanding carboxylic acids. In contrast, 4c, which is prepared from a 1:2 molar mixture of B(OH)3 and tetrachlorocatechol, is effective as a Lewis acid-assisted Bronsted acid (LBA) catalyst for Ritter reaction.
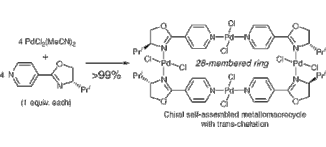
▼Design of Chiral Macrocyclic Complexes Based on trans-Chelation of n:n Metal-Bidentate P,N- or N,N-Ligands
Manabu Hatano
Takafumi Asai
Kazuaki Ishihara*
Chem. Lett. 2006, 35, 172-173.
▷http://dx.doi.org/10.1246/cl.2006.172
Chiral self-assembled macrocyclic Pd(II) and Cu(II) complexes with trans-chelation of n:n metal?bidentate P,N- and N,N-ligands have been designed toward the possibility of asymmetric Diels-Alder catalysis. Interesingly, a variety of self-assembled Pd(II) or Cu(II) complexes were obtained almost quantitatively accompanying with no other isomers, and the trans-structures of macrocyclic Pd(II) and Cu(II) complexes were determined by X-ray analysis.







