Publication 2005

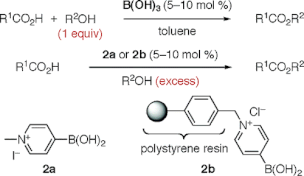
▼N-Alkyl-4-boronopyridinium Halides versus Boric Acid as Catalysts for the Esterification of -Hydroxycarboxylic Acids
Toshikatsu Maki
Kazuaki Ishihara*
Hisashi Yamamoto*
Org. Lett. 2005, 7(22), 5047-5050.
Boric acid is a highly effective catalyst for the dehydrative esterification reaction between equimolar mixtures of -hydroxycarboxylic acids and alcohols. In contrast, N-methyl-4-boronopyridinium iodide (2a) is a more effective catalyst than boric acid for the similar esterification in excess alcohol. A heterogeneous catalyst, such as N-polystyrene-bound 4-boronopyridinium chloride, is also an effective catalyst and can be recovered by filtration.
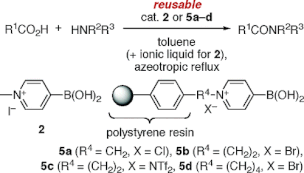
▼N-Alkyl-4-boronopyridinium Salts as Thermally Stable and Reusable Amide Condensation Catalysts
Toshikatsu Maki
Kazuaki Ishihara*
Hisashi Yamamoto*
Org. Lett. 2005, 7(22), 5043-5046.
N-Alkyl-4-boronopyridinium salts are highly effective and reusable catalysts for the dehydrative amide condensation reaction between equimolar mixtures of carboxylic acids and amines. N-Alkylboronopyridinium salts are thermally stabilized in the order N-alkyl-2-boronopyridinium salt N-alkyl-3-boronopyridinium salt < N-alkyl-4-boronopyridinium salt. Homogeneous catalysts, such as 4-borono-N-methylpyridinium iodide, are more effective in the presence of ionic liquid and can be recovered by extraction with ionic liquid. In contrast, heterogeneous catalysts, such as polystyrene-bound 4-boronopyridinium salts, are effective even in the absence of ionic liquid and can be recovered by filtration.

▼Bulky Diarylammonium Arenesulfonates as Mild and Extremely Active Dehydrative Ester Condensation Catalysts
Akira Sakakura
Shoko Nakagawa
Kazuaki Ishihara*
Tetrahedron 2006, 62, 422-433.
▷http://dx.doi.org/10.1016/j.tet.2005.09.059
More environmentally benign alternatives to current chemical processes, especially large-scale, fundamental reactions like ester condensations, are highly desirable for many reactions. Bulky diarylammonium pentafluorobenzenesulfonates and tosylates serve as extremely active dehydration catalysts for the ester condensation reaction of carboxylic acids with equimolar amounts of sterically demanding alcohols and acid-sensitive alcohols. Typically, the esterification reaction is performed in heptane by heating at 80 °C in the presence of 1 mol% of the catalyst without removing water. Esterification with primary alcohols proceeds without solvents even at room temperature. Furthermore, 4-(N-mesitylamino)polystyrene resin-bound pentafluorobenzenesulfonate can be recycled more than 10 times without a loss of activity.

▼Enantioselective Addition of Organozinc Reagents to Aldehydes Catalyzed by 3,3-Bis(diphenylphosphinoyl)-BINOL
Manabu Hatano
Takashi Miyamoto
Kazuaki Ishihara*
Adv. Synth. Catal. 2005, 347, 1561-1568.
The enantioselective addition of organozinc reagents to aromatic and aliphatic aldehydes 1 gives secondary alcohols 2 with excellent enantioselectivities in high yields through the catalytic use of (R)-3,3-bis(diphenylphosphinoyl)-BINOL (3) or (R)-3,3-bis(diphenylthiophosphinoyl)-BINOL (4) without Ti(IV) complexes. The coordination of the O or S atom of a (thio)phosphinoyl group bearing a BINOL backbone to organozinc reagents can efficiently increase the nucleophilicity of the organozinc reagents.

▼Cyanuric Chloride as a Mild and Active Beckmann Rearrangement Catalyst
Yoshiro Furuya
Kazuaki Ishihara*
Hisashi Yamamoto*
J. Am. Chem. Soc. 2005, 127, 11240-11241.
The first general organocatalytic Beckmann rearrangement of ketoximes into amides has been realized by the catalytic use of cyanuric chloride. Furthermore, acids such as HCl and ZnCl2 are effective as cocatalysts with cyanuric chloride. For example, azacyclotridecan-2-one, which is synthetically useful as a starting material for nylon-12, was prepared in quantitative yield by the Beckmann rearrangement of cyclododecanone oxime (100 mmol scale) catalyzed by cyanuric chloride (0.5 mol %) and ZnCl2 (1 mol %).

▼Chiral Lithium Binaphtholate Aqua Complex as a Highly Effective Asymmetric Catalyst for Cyanohydrin Synthesis
Manabu Hatano
Takumi Ikeno
Takashi Miyamoto
Kazuaki Ishihara*
J. Am. Chem. Soc. 2005, 127, 10776-10777.
A highly enantioselective cyanohydrin synthesis with aromatic aldehydes using chiral lithium binaphtholate aqua or alcohol complexes has been developed and is a simple and inexpensive catalyst suitable for process chemistry to give gram-scale cyanohydrins successfully. Dramatic improvements in enantiomeric excess have been realized along with an interesting changeover in absolute stereochemistry of cyanohydrin product against the thoroughly "dry" catalytic systems.
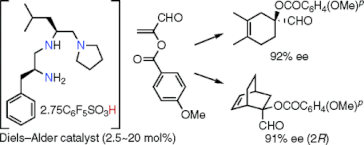
▼Design of an Organocatalyst for the Enantioselective Diels-Alder Reaction with -Acyloxyacroleins
Kazuaki Ishihara*
Kazuhiko Nakano
J. Am. Chem. Soc. 2005, 127, 10504-10505.
We have realized the first enantioselective organocatalytic Diels-Alder reaction between -substituted acroleins, such as -acyloxyacroleins, and not only cyclic but also acyclic dienes. -Acyloxyacroleins are useful as synthetic equivalents of -haloacroleins. The present catalyst could be prepared in situ from pentafluorobenzenesulfonic acid (2.5-3.0 equiv) and chiral triamine (1 equiv) derived from H-L-Phe-L-Leu-N(CH2CH2)2. The enantioselective Diels-Alder reaction of 5-(benzyloxymethyl)cyclopentadiene, cyclopentadiene, cyclohexadiene, 2,3-dimethylbutadiene, and isoprene with -(p-methoxybenzoyloxy)acrolein catalyzed by the above chiral ammonium salt (2.5-20 mol %) at -20-22 C gave the corresponding adducts with 83, 83, 91, 92, and 88% ee, respectively.

▼Facile Synthesis of Aryl- and Alkyl-bis(trifluoromethylsulfonyl)methanes
Aiko Hasegawa
Takuo Ishikawa
Kazuaki Ishihara*
Hisashi Yamamoto*
Bull. Chem. Soc. Jpn. 2005, 78, 1401-1410.
▷http://dx.doi.org/10.1246/bcsj.78.1401
Various arylbis(trifluoromethylsulfonyl)methanes (1) have been synthesized by reacting the corresponding benzylic halides with sodium trifluoromethanesulfinate and then with triflic anhydride. In addition, when the aryl group of 1 is a pentafluorophenyl group, the nucleophilic para-substitution of the aryl group with alkyllithiums and sodium alkoxides occurs. This reaction is useful for the design of new Bronsted acids.
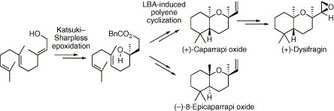
▼Biomimetic Synthesis of Acid-Sensitive (-)- and (+)-Caparrapi Oxides, (-)- and (+)-8-Epicaparrapi Oxides, and (+)-Dysifragin Induced by Artificial Cyclases
Muhammet Uyanik
Kazuaki Ishihara*
Hisashi Yamamoto*
Bioorg. Med. Chem. 2005, 13, 5055-5065.
▷http://dx.doi.org/10.1016/j.bmc.2005.04.029
Asymmetric total syntheses of acid-sensitive (-)- and (+)-caparrapi oxides (1) and (+)-8-epicaparrapi oxide (2) from farnesol (10) are achieved using Sharpless?Katsuki epoxidation and Lewis acid-assisted chiral Bronsted acid (chiral LBA)-induced polyene cyclization as key steps. The relative configuration of (+)-dysifragin (4) is determined by a single-crystal X-ray diffraction and its total synthesis is accomplished by the diastereoselective epoxidation of (+)-1. Furthermore, (-)-1 can be directly synthesized from (S)-nerolidol (3) and (R)-LBA with 88% ds by reagent control, which overcame substrate control, while (-)-2 is obtained from (R)-3 and (R)-LBA with >99% ds by the double asymmetric induction.
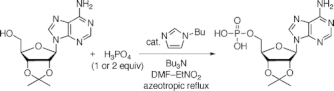
▼Selective Synthesis of Phosphate Monoesters by Dehydrative Condensation of Phosphoric Acid and Alcohols Promoted by Nucleophilic Bases
Akira Sakakura
Mikimoto Katsukawa
Kazuaki Ishihara*
Org. Lett. 2005, 7, 1999-2002.
▷DOI: 10.1021/ol0504796
Phosphate monoesters are synthesized from a mixture of phosphoric acid (1 or 2 equiv) and alcohols (1 equiv) in the presence of tributylamine. The reaction is promoted by nucleophilic bases such as N-alkylimidazole and 4-(N,N-dialkylamino)pyridine. 2',3'-O-Isopropylidene ribonucleosides are selectively converted to their 5'-monophosphates without the protection of amino groups in nucleobases.

▼Molybdenum Oxides as Highly Effective Dehydrative Cyclization Catalysts for the Synthesis of Oxazolines and Thiazolines
Akira Sakakura
Rei Kondo
Kazuaki Ishihara*
Org. Lett. 2005, 7, 1971-1994.
▷DOI: 10.1021/ol050543j
In the presence of molybdenum oxide the dehydrative cyclization of N-acylserines, N-acylthreonines, and N-acylcysteines can be carried out under Dean-Stark conditions in toluene to give oxazolines and thiazolines. The ammonium salts (NH4)6Mo7O24・4H2O and (NH4)2MoO4 have excellent catalytic activities for the dehydrative cyclization of serine and threonine derivatives, and the acetylacetonate complex MoO2(acac)2 has a remarkable catalytic activity for the dehydrative cyclization of cysteine derivatives. In addition, polyaniline-supported MoO2(acac)2 can easily be recovered and reused.
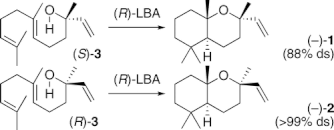
▼Biomimetic Synthesis of Acid-Sensitive (-)-Caparrapi Oxide and (+)-8-Epicaparrapi Oxide Induced by Artificial Cyclases
Muhammet Uyanik
Hideaki Ishibashi
Kazuaki Ishihara*
Hisashi Yamamoto*
Org. Lett. 2005, 7, 1601-1604.
▷DOI: 10.1021/ol050295r
Asymmetric total syntheses of acid-sensitive (-)-caparrapi oxide (1) and (+)-8-epicaparrapi oxide (2) from farnesol (9) were achieved using Sharpless-Katsuki epoxidation and Lewis acid-assisted chiral Brensted acid (chiral LBA)-induced polyene cyclization as key steps. Furthermore, (-)-1 could be directly synthesized from (S)-nerolidol (3) and (R)-LBA with 88% ds by reagent control which overcame substrate control, while (-)-2 was obtained from (R)-3 and (R)-LBA with >99% ds by the double-asymmetric induction.

▼Bulky Diarylammonium Arenesulfonates as Selective Esterification Catalysts
Kazuaki Ishihara*
Shoko Nakagawa
Akira Sakakura
J. Am. Chem. Soc. 2005, 127, 4168-4169.
▷DOI: 10.1021/ja050223v
More environmentally benign alternatives to current chemical processes, especially large-scale, fundamental reactions such as ester condensations, are highly desirable for many reactions. Bulky diarylammonium pentafluorobenzenesulfonates and tosylates serve as extremely active dehydration catalysts for the ester condensation reaction of carboxylic acids with equimolar amounts of sterically demanding alcohols and acid-sensitive alcohols. Typically, the esterification reaction is performed in heptane by heating at 80 C in the presence of 1 mol % of the catalyst without removing water. Esterification with primary alcohols proceeds without solvents even at room temperature. Furthermore, 4-(N-mesitylamino)polystyrene resin-bound pentafluorobenzenesulfonate can be recycled more than 10 times without activity loss.

▼Synthesis of (all-rac)-a-Tocopherol in Supercritical Carbon Dioxide: Tuning of the Product Selectivity in Batch and Continuous-Flow Reactors"
Yoshiaki Kokubo
Aiko Hasegawa
Shigeki Kuwata
Kazuaki Ishihara
Hisashi Yamamoto
Takao Ikariya*
Adv. Synth. Catal. 2005, 347, 220-224.
▷DOI: 10.1002/adsc.200404312
a-Tocopherol was synthesized using a condensation reaction of 2,3,6-trimethylhydroquinone with isophytol in supercritical CO2 using batch and continuous-flow reactors. In the batch reaction catalyzed by a fluorinated molecular catalyst bearing strong Bronsted acidity, C6F5CHTf2 (Tf=SO2CF3), an increase in the CO2 pressure causes a marked increase in the product selectivity for a-tocopherol, albeit with a slight decrease in the product yield. The solubility measurements by extraction experiments and the supercritical fluid NMR (scNMR) indicate that the homogeneous and non-polar reaction phase in scCO2 is crucial to obtain a-tocopherol with high selectivity. A continuous flow scCO2 process for the condensation reaction can be performed with a strong acid resin, Amberlyst 15, as a solid acid catalyst to give the desired product with high selectivity.

▼Highly Alkyl-Selective Addition to Ketones with Magnesium Ate Complexes Derived from Grignard Reagents
Manabu Hatano
Tokihiko Matsumura
Kazuaki Ishihara*
Org. Lett. 2005, 7, 573-576.
▷DOI:
10.1021/ol047685i
A highly efficient alkyl-selective addition to ketones with magnesium ate complexes derived from Grignard reagents and alkyllithiums is described. The nucleophilicity of R in R3MgLi is remarkably increased compared to that of the original RLi or RMgX, while the basicity of R3MgLi is decreased. Furthermore, a highly R-selective addition to ketones is demonstrated using RMe2MgLi in place of R3MgLi.







