Ishihara World: Rational Design of High-Performance Catalysts
Based on Acid–Base Combination Chemistry
Top News




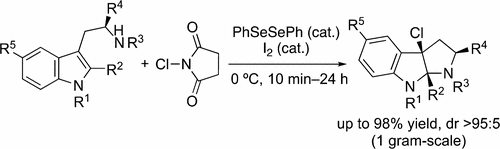
▼Selenium–Iodine Cooperative Catalyst for Chlorocyclization of Tryptamine Derivatives
Org. Lett. 2017, ASAP.
DOI: 10.1021/acs.orglett.7b02613
▼Chiral Hypervalent Organoiodine-Catalyzed Enantioselective Oxidative Spirolactonization of Naphthol Derivatives
Muhammet Uyanik, Takeshi Yasui, Kazuaki Ishihara*

▼Enantioselective Conjugate Hydrocyanation of α,β-Unsaturated N-Acylpyrroles Catalyzed by Chiral Lithium(I) Phosphoryl Phenoxide
Enantioselective conjugate hydrocyanation of α,β-unsaturated N-acylpyrroles with the combined use of Me3SiCN, LiCN, and HCN has been developed in the presence of a chiral lithium(I) phosphoryl phenoxide catalyst. This reaction is useful for a variety of N-acylpyrroles, including previously unreported substrates, such as heteroaryl and halogen-substituted N-cinnamoylpyrroles. A gram-scale reaction and subsequent transformations to a (R)-succinate, (S)-paraconic acid, and (R)-baclofen demonstrate an entry for the practical synthesis of optically active β-substituted γ-aminobutyric acids (GABA).
▼Cover Feature: Design of Boronic Acid–Base Complexes as Reusable Homogeneous Catalysts in Dehydrative Condensations between Carboxylic Acids and Amines
Yanhui Lu, Ke Wang and Kazuaki Ishihara*

▼Design of Boronic Acid–Base Complexes as Reusable Homogeneous Catalysts in Dehydrative Condensations between Carboxylic Acids and Amines
Yanhui Lu, Ke Wang, and Kazuaki Ishihara*

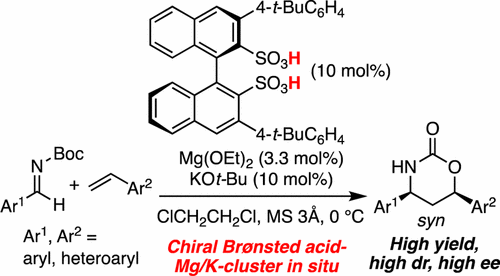
▼Enantioselective Cycloaddition of Styrenes with Aldimines Catalyzed by a Chiral Magnesium Potassium Binaphthyldisulfonate Cluster as a Chiral Brønsted Acid Catalyst
A chiral magnesium potassium binaphthyldisulfonate cluster, as a chiral Brønsted acid catalyst, was shown to catalyze an enantioselective cycloaddition of styrenes with aldimines for the first time. The strong Brønsted acidity of the catalyst precursors, which might dissolve drying agents and take up the leached Mg2+ and K+, serendipitously led to good enantioselectivity. Mechanistic aspects were supported by X-ray and ESI-MS analysis of the catalyst and a kinetics study of the reaction. Useful transformations to optically active 1,3-amino alcohols on a gram scale were also demonstrated.