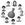Assoc. Prof. Dr. Muhammet UYANIK

Nagoya University
Graduate School of Engineering
Department of Chemistry & Biotechnology
Laboratory of Catalysis in Organic Synthesis
Associate Professor
Professional Societies:
The Chemical Society of Japan (CSJ)
The Society of Synthetic Organic Chemistry, Japan (SSOCJ)
The Society of Iodine Sciences, Japan (SIS)
muha[at]chembio.nagoya-u.ac.jp
TEL +81-52-789-3206 / FAX +81-52-789-3222
Muha Group Member (2024)
Kai Matsui (D3), Shunsuke Minabe (D1), Yaohan Liang (D1), Kotaro Asada (M2), Takaharu Morino (M2), Sho Akagawa (M1), Masato Tsukamoto (M1), Sari Arikawa (B4), Ryusuke Mashita (B4), Takahito Mizuno (B4)
Alumni
Hiroaki Okamoto (2011), Ryota Fukatsu (2011), Erina Kaneko (2011), Daisuke Nakashima (2012), Dr. Takeshi Yasui (2013), Kento Ohori (2013), Dai Nagata (2014), Dr. Daisuke Suzuki (2015),
Dr. Niiha Sasakura (2015), Mayuko Tsukahara (2015), Dr. Hiroki Hayashi (2016), Masahiro Mizuno (2016), Yuhei Hattori (2016), Fumio Nakashima (2016), Dr. Tatsuya Mutsuga (2017),
Naoya Ukegawa (2017), Kirara Saeda (2017), Hirokazu Iwata (2018), Dr. Kohei Nishioka (2019), Outa Katade (2019), Dr. Naoto Sahara (2020), Ryutaro Kondo (2020), Shinichi Ishizaki (2021), Toshihiro Yasui (2021), Soki Ihara (2021), Yuya Okada (2021), Dr. Hiroki Tanaka (2022), Sachiko Kumagai (2022), Yasuo Tsukimori (2022), Dr. Takehiro Kato (2023), Shogo Yamamoto (2023), Yuto Fujii (2024), Shunki Matsuyama (2024),
Education/Academic Experience
1981 Born in Asarcik, Samsun, Turkey
2000 Tokyo Gakuyukai International Japanese Language School, Graduated
2004 School of Engineering Nagoya University, Graduated
2006 Mr. of Eng., Department of Biotechnology, Nagoya University (Advisor: Prof. Kazuaki Ishihara)
2007 Dr. of Eng., Department of Biotechnology, Nagoya University (Advisor: Prof. Kazuaki Ishihara)
Thesis: “Development of Biomimetic Polyene Cyclization Directed towards Total Synthesis of Polycyclic Terpenoids”
2004-2007 Japanese Government (Monbukagakusho) Scholarship Student
2007 Assistant Professor, Graduate School of Engineering, Nagoya University
2020 Associate Professor, Graduate School of Engineering, Nagoya University
Awards
1998 9th International Biology Olympiad, Germany, Bronze Medal
2008 Green & Sustainable Chemistry Poster Award
2008 Best Presentation Award (Academic), 88th Annual Meeting of CSJ
2009 Shionogi Award in Synthetic Organic Chemistry from SSOCJ
2009 Best Presentation Award (Academic), 89th Annual Meeting of CSJ
2010 Incentive Award for Young Scientist, Tokai Branch of SSOCJ
2011 Akazaki Prize, Nagoya University
2012 The Grand Prize of 6th Wakashachi Incentive Award, Aichi Prefectural Government
2012 The Young Scholar’s Lectureship Award, 92nd Annual Spring Meeting of CSJ
2012 The 15th Symposium on the Society of Iodine Science Poster Award
2013 The Young Scientists’ Prize (The Commendation for Science and Technology by MEXT)
2015 7th Inoue Science Research Award
2015 The 4th GSC Encouragement Award
2015 Banyu Chemist Award (BCA)
2016 Thieme Chemistry Journal Award
2017 The Chemical Society of Japan Award for Young Chemists
2022 Asian Core Program / Advanced Research Network Lectureship Award (Hong Kong)
Professional Activities
2013–16 Turkish Journal of Chemistry, Editorial Board
2014–16 Journal of Synthetic Organic Chemistry, Japan, Associate Member of Editorial Board
2014 The 4th Int. Conference on Hypervalent Iodine Chem. (ICHIC2014), Organizing Committee
2017 The 8th Int. Meeting on Halogen Chem. (HALCHEM VIII), Organizing Committee
Publication
70. Electrochemical oxidative dearomatization of electron-deficient phenols using Br+/Br– catalysis
Kai Matsui, Muhammet Uyanik,* Kazuaki Ishihara*
Chem. Commun. 2025, 61, 2075–2078.

69. Unraveling Alcohol Additive Effects on Hypervalent Iodine(III)-Catalyzed Asymmetric Phenolic Dearomatization: Ligand Substitution and Low-Barrier Hydrogen Bonds
Hanliang Zheng,* Liu Cai, Xiaoyu Lai, Muhammet Uyanik, Kazuaki Ishihara, and Xiao-Song Xue*
ACS Catal. 2025, 15, 370–380.
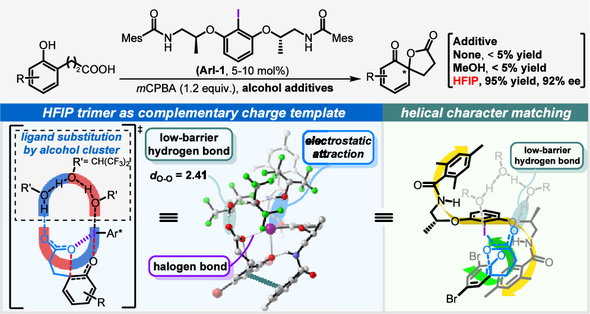
68. Inner-Bond-Cleavage Approach to Figure-Eight Macrocycles from Planar Aromatic Hydrocarbons
Reiji Yoshina, Junichiro Hirano, Emiko Nishimoto, Yuki Sakamoto, Keita Tajima, Shunsuke Minabe, Muhammet Uyanik, Kazuaki Ishihara, Tomoyuki Ikai, Eiji Yashima, Takuya Omine, Fumitaka Ishiwari, Akinori Saeki, Jinseok Kim, Juwon Oh, Dongho Kim, Guanting Liu, Takuma Yasuda, Hiroshi Shinokubo, and Norihito Fukui*
J. Am. Chem. Soc. 2024, 146, 29383−29390.
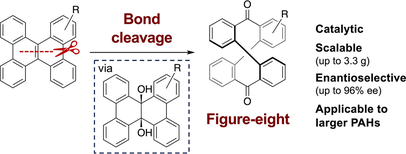

67. Oxidative Dearomative Coupling of Electron-Deficient Arenols Using Hypohalite Catalysis
Takehiro Kato, Naoto Sahara, Sho Akagawa, Muhammet Uyanik*, Kazuaki Ishihara*
Org. Lett. 2024, 26, 7255–7260.

66. Oxidation: Asymmetric Oxidative Dearomatization
Muhammet Uyanik*, Kazuaki Ishihara*
In Comprehensive Chirality, 2nd edition, Cossy, J., Ed., Elsevier: Academic Press, 2024, pp 243–265. ISBN: 978-0-323-90645-6.
65. (2R,2′R)-2,2′-[(2-Iodo-1,3-phenylene)bis(oxy)]bis[N-(2,4,6-trimethylphenyl)propanamide]
Muhammet Uyanik*, Kazuaki Ishihara*
In Electronic Encyclopedia of Reagents for Organic Synthesis (e-EROS), John Wiley & Sons, 2024.

64. Hypoiodite-Catalyzed Oxidative α-C−N Coupling of Ketones with Imides and Azoles
Mayuko Tsukahara, Muhammet Uyanik*, Kazuaki Ishihara*
Adv. Synth. Catal. 2023,365, 2724–2729.
- Selected as VIP (Very Important Publication).
- Special Issue: Iodine in Catalysis and Organic Synthesis

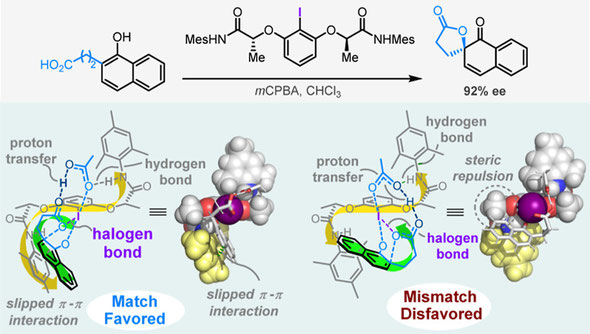
63. Catalyst-Substrate Helical Character Matching Determines the Enantioselectivity in the Ishihara-Type Iodoarenes Catalyzed Asymmetric Kita-Dearomative Spirolactonization
Hanliang Zheng, Liu Cai, Ming Pan, Muhammet Uyanik, Kazuaki Ishihara, Xiao-Song Xue*
J. Am. Chem. Soc. 2023, 145, 13, 7301–7312.
62. 1,3-Migrative Ring Expansion of Spiroindolenines to Azepino[3,4-b]indoles
Hiroki Tanaka, Toshihiro Yasui, Muhammet Uyanik*, and Kazuaki Ishihara*
Org. Lett. 2023, 25, 14, 2377–2381.
DOI: 10.1021/acs.orglett.3c00207
- Selected as Front Cover (Issue 14).


61. Chiral Ammonium Hypoiodite Catalysis for Enantioselective Oxidative Dearomatization Reactions
Muhammet Uyanik,* Kazuaki Ishihara*
月刊ファインケミカル 2022, 51(8), 31–39.
60. Asymmetric Hypervalent Iodine Catalysis
Muhammet Uyanik,* Kazuaki Ishihara*
In: Catalytic Asymmetric Synthesis, 4th Ed., Takahiko Akiyama and Iwao Ojima (Eds.),
John Wiley & Sons: Hooboken, 2022, pp. 243–276. ISBN: 978-1119736424.
59. Hypoiodite-Catalyzed Oxidative Umpolung of Indoles for Enantioselective Dearomatization
Hiroki Tanaka, Naoya Ukegawa, Muhammet Uyanik*, Kazuaki Ishihara*
J. Am. Chem. Soc. 2022, 144, 5756–5761.
- Press Release from Nagoya University and 日本の研究.
- Highlighted in ChemStation.
- Highlighted in Synfacts 2022, 18, 671 ("Hypoiodite Permits Enantioselective Dearomatization of Indoles" by B. List & R. Xu).

58. Catalytic Oxidative α-Functionalization of Carbonyls
Muhammet Uyanik
In: Iodine Catalysis in Organic Synthesis, Kazuaki Ishihara and Kilian Muñiz (Eds.),
Wiley-VCH: Weinheim, 2022, pp. 275–298. ISBN: 978-3527348299.
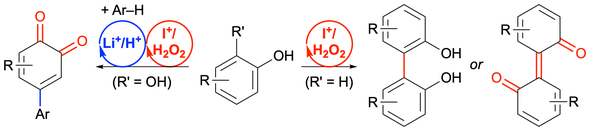
57. Hypoiodite-catalysed oxidative homocoupling of arenols and tandem oxidation/cross-coupling of hydroquinones with arenes
Muhammet Uyanik, Dai Nagata and Kazuaki Ishihara*
Chem. Commun. 2021, 57, 11625–11628.
- Selected as “Chemical Communications HOT articles".
56. Oxidative Ritter-type Chloroamidation of Alkenes Using NaCl and Oxone
Takehiro Kato, Yuya Okada, Yuto Fujii, Muhammet Uyanik*, Kazuaki Ishihara*
Asian J. Org. Chem. 2021, 10, 2907-2910. (Special Collection)

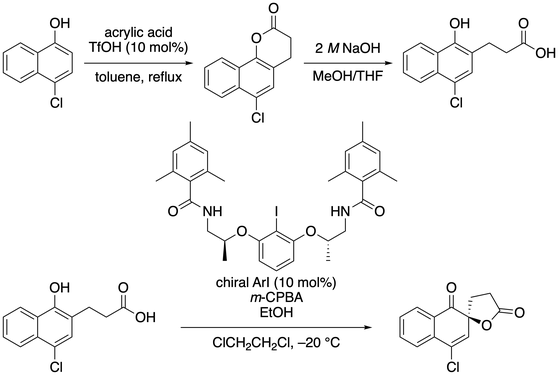
55. Chiral Organoiodine-catalyzed Enantioselective Oxidative Dearomatization of Phenols
Muhammet Uyanik, Shinichi Ishizaki, Kazuaki Ishihara* (checked by Tao Wang, Kevin Campos)
Org. Synth. 2021, 98, 28–50.
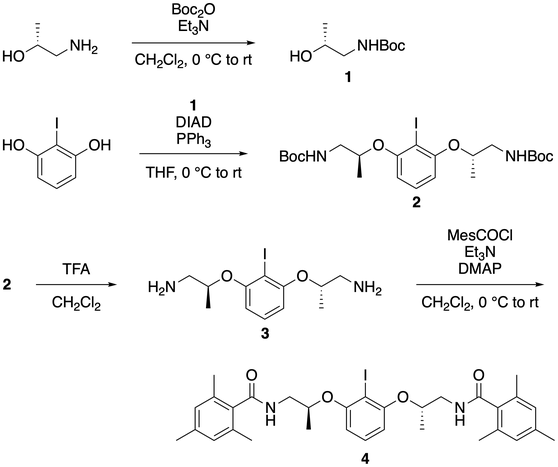
54. Synthesis of Chiral Organoiodine Catalyst for Enantioselective Oxidative Dearomatization Reactions: N,N'-(2S,2'S)-(2-Iodo-1,3-phenylene)bis(oxy)bis(propane-2,1-diyl)bis(2,4,6-trimethylbenzamide)
Muhammet Uyanik, Shinichi Ishizaki, Kazuaki Ishihara* (checked by Cayetana Zarate, Kevin Campos)
Org. Synth. 2021, 98, 1–27.
53. I+/TBHP Catalysis For Tandem Oxidative Cyclization To Indolo[2,3-b]quinolines
Muhammet Uyanik, Hiroki Tanaka, Kazuaki Ishihara*
Asian J. Org. Chem. 2021, 10, 164–169. (Special Issue: Organocatalysis)
52. 高活性次亜ヨウ素酸塩/過酸化水素触媒システムを用いるカルボニルの酸化的αーアジド化反応(High-Performance Hypoiodite/Hydrogen Peroxide Catalysis for Oxidative α-Azidation of Carbonyls)
Muhammet Uyanik,* Naoto Sahara, Mayuko Tsukahara, Yuhei Hattori, Kazuaki Ishihara*
SIS Report 2020, 23, 10–13.
51. Hypoiodite-Catalyzed Chemoselective Tandem Oxidation of Homotryptamines to Peroxy- and Epoxytetrahydropyridoindolenines
Muhammet Uyanik, Hiroki Tanaka, Kazuaki Ishihara*
Org. Lett. 2020, 22, 8049–8054.

50. Chemo‐ and Enantioselective Oxidative α-Azidation of Carbonyl Compounds
Muhammet Uyanik, Naoto Sahara, Mayuko Tsukahara, Yuhei Hattori, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2020, 59, 17110–17117; Angew. Chem. 2020, 132, 17258–17265.
- Press Release from Nagoya University and 日本の研究.
- Highlighted in Japanese Newspapers (Kagaku Kogyo Nippo).
- Highlighted in Synfacts 2020, 16, 1094 ("Hypoiodite Catalysis Permits a General Oxidative Azidation of 1,3-Dicarbonyl Compounds" by B. List & B. Mitschke).
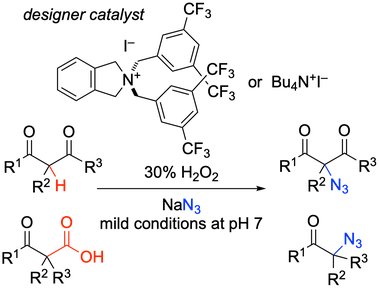
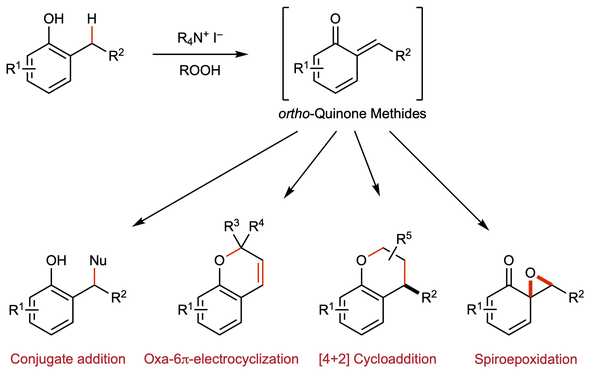
49. Chemoselective Oxidative Generation of ortho-Quinone Methides and Tandem Transformations
Muhammet Uyanik, Kohei Nishioka, Ryutaro Kondo, Kazuaki Ishihara*
Nature Chem. 2020, 12, 353–362.
DOI: 10.1038/s41557-020-0433-4
- News&Views "Mild map to quinone methides" by Boris J. Nachtsheim in Nature Chem.
- Press Release from Nagoya University and 日本の研究.
- Highlighted in Japanese Newspapers (Kagaku Kogyo Nippo, Kagaku Shinbun, Mainichi Shinbun).
- Highlighted in Synfacts 2020, 16, 708 ("A Transition Metal-Free Catalytic Approach Towards Selective Transformations of ortho-Quinone Methides" by B. List & B. Mitschke).
- Highlighted in Journal of Synthetic Organic Chemistry, Japan 2021, 79(1), p83.
48. Chemoselective Oxidative Spiroetherification and Spiroamination of Arenols Using I+/Oxone Catalysis
Muhammet Uyanik, Naoto Sahara, Outa Katade, Kazuaki Ishihara*
Org. Lett. 2020, 22, 560–564.

46. Designer C2-Symmetric Chiral Diamide-Type Organoiodine Catalysts
Muhammet Uyanik,* Kazuaki Ishihara*
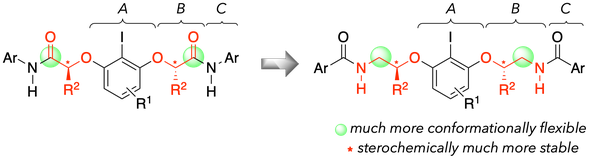
45. High-Performance Ammonium Hypoiodite/Oxone Catalysis for Enantioselective Oxidative Dearomatization of Arenols
Muhammet Uyanik, Takehiro Kato, Naoto Sahara, Outa Katade, Kazuaki Ishihara*
ACS Catal. 2019, 9, 11619–11626.
- Featured in Org. Chem. Highlights: Organocatalyzed C-C Ring Construction.
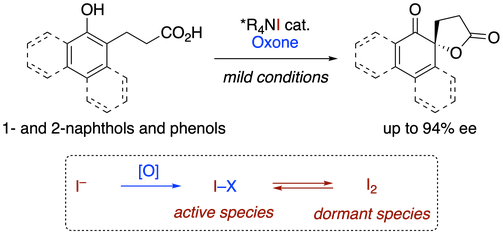
44. Ammonium Hypoiodite-catalyzed Oxidative Dearomatizative Azidation of Arenols
Muhammet Uyanik, Kohei Nishioka, Kazuaki Ishihara*
Chem. Lett. 2019, 48, 353–356.

43. Regioselective Oxidative Chlorination of Arenols Using NaCl and Oxone
Muhammet Uyanik, Naoto Sahara, Kazuaki Ishihara*
Eur. J. Org. Chem. 2019, 27–31.
- Selected as Very Important Paper (VIP) and Front Cover.


42. Oxidation of Alcohols and Amines
Muhammet Uyanik,* Kazuaki Ishihara*
In: Patai's Chemistry of Functional Groups, Ilan Marek, Berit Olofsson and Zvi Rappoport (Eds.), John Wiley & Sons: Chichester, 2019, pp. 261-306. ISBN: 978-1119352303.
41. Asymmetric Total Synthesis of (−)-Maldoxin, a Common Biosynthetic Ancestor of the Chloropupukeananin Family
Takahiro Suzuki,* Soichiro Watanabe, Muhammet Uyanik, Kazuaki Ishihara, Susumu Kobayashi, Keiji Tanino
Org. Lett. 2018, 20, 3919–3922.

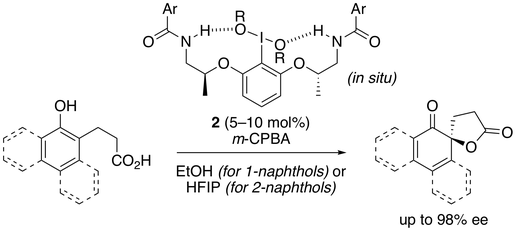
40. Chiral Hypervalent Organoiodine-Catalyzed Enantioselective Oxidative Spirolactonization of Naphthol Derivatives
Muhammet Uyanik, Takeshi Yasui, Kazuaki Ishihara*
J. Org. Chem. 2017, 82, 11946–11953.
-
Special Issue: Hypervalent
Iodine Reagents
39. α-Oxidation of Carbonyl Compounds
Muhammet Uyanik,* Kazuaki Ishihara*
In: Science of Synthesis: Catalytic Oxidation in Organic Synthesis, Kilian Muniz (ed.), Georg Thieme-Verlag: Stuttgart, 2017, pp. 635–670. ISBN: 978-3132012417.
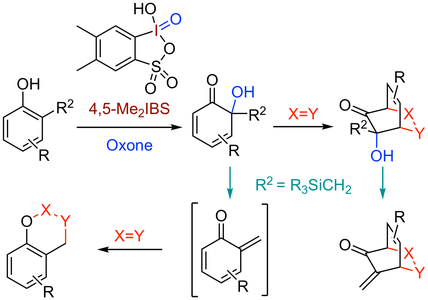
38. 4,5-Dimethyl-2-Iodoxybenzenesulfonic Acid Catalyzed Site-Selective Oxidation of 2-Substituted Phenols to 1,2-Quinols
Muhammet Uyanik, Tatsuya Mutsuga, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2017, 56, 3956–3960; Angew. Chem. 2017, 129, 4014–4018.
37. Enantioselective Synthesis of Masked Benzoquinones Using Designer Chiral Hypervalent Organoiodine(III) Catalysis
Muhammet Uyanik, Niiha Sasakura, Masahiro Mizuno, Kazuaki Ishihara*
ACS Catal. 2017, 7, 872–876.
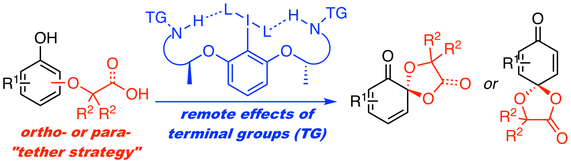
36. Ammonium Hypoiodite-catalyzed Peroxidative Dearomatization of Phenols
Muhammet Uyanik, Kohei Nishioka, Kazuaki Ishihara*
Heterocycles 2017, 95, 1132–1147.
- Special Issue in honor of Professor Dr. Masakatsu Shibasaki on 70th Birthday.

35. Chiral Quaternary Ammonium Hypoiodite Catalysis
Muhammet Uyanik,* Kazuaki Ishihara*
月刊ファインケミカル 2016, 45(12), 16–24.
34. Asymmetric Oxidative Dearomatization Reaction
Muhammet Uyanik,* Kazuaki Ishihara*
In: Asymmetric Dearomatization Reactions, Shu-Li You (ed.), Wiley-VCH: Weinheim, 2016, pp. 129–152. ISBN: 978-3527338511.
33. Chiral Ammonium Hypoiodite Salt-Catalyzed Enantioselective Oxidative Cycloetherification to 2-Acyl Tetrahydrofurans
Muhammet Uyanik, Hiroki Hayashi, Hirokazu Iwata, Kazuaki Ishihara*
Chem. Lett. 2016, 45, 353–355.
- Featured in Org. Chem. Highlights: Organocatalyzed C-O Ring Construction.

32. Practical Oxidative Dearomatization of Phenols with Sodium Hypochlorite Pentahydrate
Muhammet Uyanik, Niiha Sasakura, Mitsuyoshi Kuwahata, Yasukazu Ejima, Kazuaki Ishihara*
Chem. Lett. 2015, 44, 381–383.

31. High-Performance Hypoiodite/Hydrogen Peroxide Catalytic System for the Oxylactonization of Aliphatic γ-Oxocarboxylic Acids
Muhammet Uyanik, Daisuke Suzuki, Mizu Watanabe, Hiroyasu Tanaka, Kikuo Furukawa, Kazuaki Ishihara*
Chem. Lett. 2015, 44, 387–389.

30. Chiral Ammonium Hypoiodite-catalyzed Enantioselective Oxidative Dearomatization of 1-Naphthols Using Hydrogen Peroxide
Muhammet Uyanik, Niiha Sasakura, Erina Kaneko, Kento Ohori, Kazuaki Ishihara*
Chem. Lett. 2015, 44, 179–181.
- Selected as Editor’s Choice

29. High-Turnover Hypoiodite Catalysis for Asymmetric Synthesis of Tocopherols
Muhammet Uyanik, Hiroki Hayashi, Kazuaki Ishihara*
Science 2014, 345, 291–294.
- Perspective by Boris J. Nachtsheim in Science.
-
Press Release from Nagoya
University and JST.
- Highlighted in Chem. & Eng. News, ACS (July 21, 2014, p8).
- Highlighted in Japanese Newspapers (Chunichi, Asahi, Nikkan Kogyo, Kagaku Kogyo Nippo, Kagaku Shinbun, etc.).
-
Highlighted in ChemStation.
- Highlighted in Synfacts 2014, 10, 1093 ("Enantioselective Synthesis of Tocopherols Through Oxidative Cyclization" by B. List & G. A. ).
- Highlighted in Angew. Chem. Int. Ed. 2014 ("Building Up Quarternary Stereocenters of Chromans by Asymmetric Redox Organocatalysis: A New Entry to Vitamin E" by T. Netscher).

28. Functional Group Transformations via Carbonyl Derivatives
Muhammet Uyanik,* Kazuaki Ishihara*
In: Comprehensive Organic Synthesis, 2nd Edition, Gary A. Molander and Paul Knochel (eds.), Vol. 6, Elsevier: Oxford, 2014, pp. 573–597. ISBN: 978-0080977423.
27. 2-ヨードベンゼンスルホン酸(pre-IBS)とOxoneを用いる選択的酸化反応
Muhammet Uyanik, Tatsuya Mutsuga, Kazuaki Ishihara*

26. Hydrogen Bonding and Alcohol Effects in Asymmetric Hypervalent Iodine Catalysis: Enantioselective Oxidative Dearomatization of Phenols
Muhammet Uyanik, Takeshi Yasui, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2013, 52, 9215–9218; Angew. Chem. 2013, 125, 9385–9388.
- Highlighted in Japanese Newspapers (Chunichi, Kagaku Kogyo Nippo).
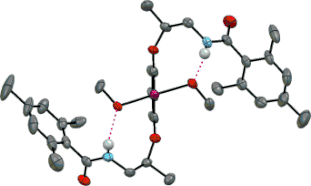
25. Baeyer−Villiger Oxidation Using Hydrogen Peroxide
Muhammet Uyanik, Kazuaki Ishihara*
ACS Catal. 2013, 3, 513–520. (Perspective)

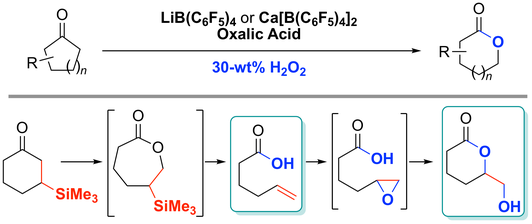
24. Baeyer−Villiger Oxidation and Oxidative Cascade Reactions with Aqueous Hydrogen Peroxide Catalyzed by Lipophilic LiB(C6F5)4 or
Ca[B(C6F5)4]2
Muhammet Uyanik, Daisuke Nakashima, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2012, 51, 9093–9096; Angew. Chem. 2012, 124, 9227–9230.
- Highlighted in Japanese Newspapers (Chunichi, NIkkan Kogyo, Kagaku Kogyo Nippo).
- Highlighted in Chemical Engineering (October 1, 2012).
- Highlighted in Organic Chemistry Portal.
- Highlighted in Advances In Engineering.
- Featured in Org. Chem. Highlights: Functional Group Oxidation.

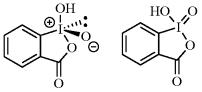
21. 1,2-Benziodoxol-3(1H)-one, 1-Hydroxy, 1-Oxide (First Update)
K. C. Nicolaou, Tamsyn Montagnon, Phil S. Baran, Muhammet Uyanik, Kazuaki Ishihara
In Catalytic Oxidation Reagents, Handbook of Reagents for Organic Chemistry Series, Philip L. Fuchs (ed.), John Wiley & Sons: Chichester, 2013, pp. 32-48. ISBN: 978-1119953272.
(Electronic Encyclopedia of Reagents for Organic Synthesis (e-EROS), John Wiley & Sons, 2012)
20. IBS-Catalyzed Regioselective Oxidation of Phenols to 1,2-Quinones with Oxone
Muhammet Uyanik, Tatsuya Mutsuga, Kazuaki Ishihara*
Molecules, 2012, 17, 8604–8616. (Special Issue: Hypervalent Compounds)

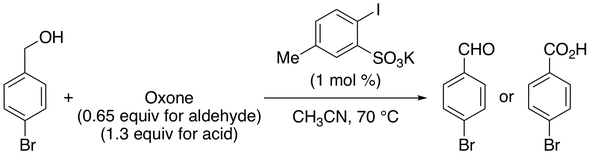
19. 2-Iodoxy-5-Methylbenzenesulfonic Acid-Catalyzed Selective Oxidation of 4-Bromobenzyl Alcohol to 4-Bromobenzaldehyde or 4-Bromobenzoic Acid with Oxone
Muhammet Uyanik, Kazuaki Ishihara* (checked by Richard Crockett, Margaret Faul)
Org. Synth. 2012, 89, 105–114.
18. Catalysis with In Situ-Generated (Hypo)iodite Ions for Oxidative Coupling Reactions
Muhammet Uyanik, Kazuaki Ishihara*
ChemCatChem 2012, 4, 177–185. (Concept Article)
- Selected as Very Important Paper (VIP).

17. In Situ Generated (Hypo)Iodite Catalysts for the Direct α-Oxyacylation of Carbonyl Compounds with Carboxylic Acids
Muhammet Uyanik, Daisuke Suzuki, Takeshi Yasui, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2011, 50, 5331–5334; Angew. Chem. 2011, 123, 5443–5446.
- Highlighted in Japanese Newspaper (Nikkan Kogyo Shinbun).
- Highlighted in Pharmacia (by Miyamoto Kazunori, 2012, 48(3), 237).
- Highlighted in TCI Topics.
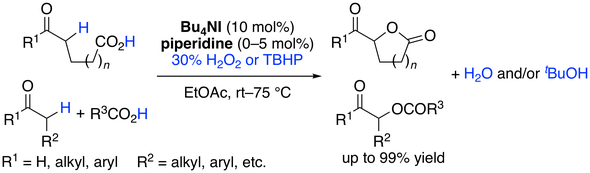
16. In Situ-Generated Chiral Quaternary Ammonium (Hypo)iodite Catalysis for Enantioselective Oxidative Cyclisations
Muhammet Uyanik, Kazuaki Ishihara*

15. 非共有結合相互作用を利用したキラル超原子価ヨウ素触媒の開発:北スピロラクトン化反応への応用
Muhammet Uyanik, Takeshi Yasui, Kazuaki Ishihara*

14. Chiral Hypervalent Iodine-Catalyzed Enantioselective Oxidative Kita Spirolactonization of 1-Naphthol Derivatives and One-Pot Diastereoselective Oxidation to Epoxyspirolactones
Muhammet Uyanik, Takeshi Yasui, Kazuaki Ishihara*
Tetrahedron 2010, 66, 5841–5851.
DOI: 10.1016/j.tet.2010.04.060
- Special Issue: Hypervalent Iodine Chemistry.
-
Highlighted in Synfacts 2010, 9, 1000 ("Catalytic Hypervalent Iodine Spirolactonization Using m-CPBA as Co-Oxidant" by ).
- Recognized by Elsevier as one of their "Top-25 most cited articles" as published in Tetrahedron for 2010-2011.

12. Quaternary Ammonium (Hypo)iodite Catalysis for Enantioselective Oxidative Cycloetherification
Muhammet Uyanik, Hiroaki Okamoto, Takeshi Yasui, Kazuaki Ishihara*
Science 2010, 328, 1376–1379.
- Perspective by Andrew N. French in Science.
-
Press Release from Nagoya
University and JST.
- Highlighted in Nature (Nature, 2010, 465, 849).
- Highlighted in Chemistry World, RSC (June 10, 2010).
- Highlighted in Chem. & Eng. News, ACS (June 14, 2010, p11).
- Highlighted in Chemistry & Industry (June 21, 2010).
- Highlighted in Chemical Engineering (September 1, 2010).
- Highlighted in Japanese Newspapers (Chunichi, Yomiuri, Asahi, Mainichi, Nikkei Sangyo, Nikkan Kogyo, Kagaku Kogyo Nippo, Kagaku Shinbun, Kyodo Press).
- Highlighted in Synform 2010, 9, A79-A83.
- Highlighted in Nagoya University Research.
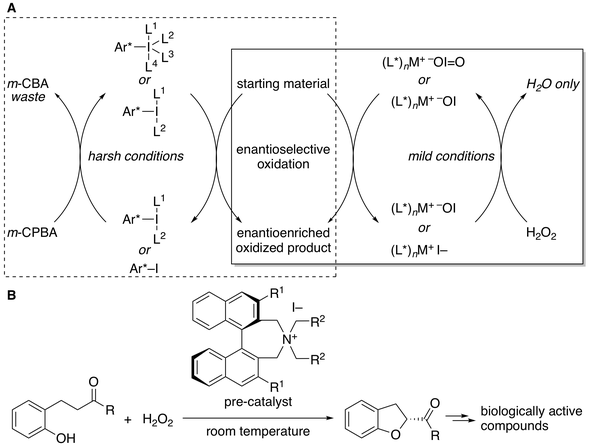
11. Enantioselective Kita Oxidative Spirolactonization Catalyzed by In Situ Generated Chiral Hypervalent Iodine(III) Species
Muhammet Uyanik, Takeshi Yasui, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2010, 49, 2175–2177; Angew. Chem. 2010, 122, 2221–2223.
- Selected as “Hot Paper” and “Front Cover” (Issue 12).
-
Press Release from Nagoya
University.
- Highlighted in Japanese Newspapers (Chunichi, Yomiuri, Mainichi, Nikkan Kogyo, Kagaku Kogyo Nippo, Jiji Press, Kyodo Press, Yahoo News, etc.).
- Rank in Most Accessed Articles (Mar 2010).
- Highlighted in Synfacts 2010, 5, 602 ("Kita’s Oxidative Spirolactonization Catalyzed by a Hypervalent Iodine(III) Species" by B. List, K. ZUmbansen).
-
Highlighted in Journal of Synthetic Organic Chemistry, Japan 2011, 69(1), p86.
- Chiral iodoarene pre-catalyst [(2R,2R’)-2,2’-(2-iodo-1,3-phenylene)bis(oxy)bis(N-mesitylpropanamine)] is commercially available from Wako Pure Chemical Industries, Ltd. and TCI Co., Ltd.
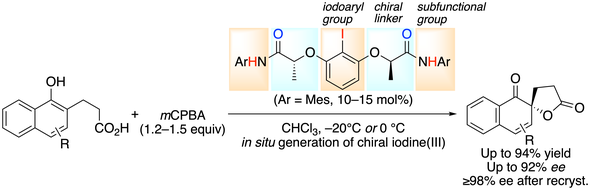

10. 2-Iodoxybenzenesulfonic Acid (IBS)-Catalyzed Oxidation of Alcohols
Muhammet Uyanik, Kazuaki Ishihara*
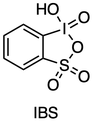
9. 2-Iodobenzenesulfonic Acid
Muhammet Uyanik, Kazuaki Ishihara, Chi Zhang, Li-Qian Cui
In Catalytic Oxidation Reagents, Handbook of Reagents for Organic Chemistry Series, Philip L. Fuchs (ed.), John Wiley & Sons: Chichester, 2013, pp. 332-338. ISBN: 978-1119953272. (Electronic Encyclopedia of Reagents for Organic Synthesis (e-EROS), John Wiley & Sons, 2010)

8. Bromine Catalyzed Aerobic Oxidation of Alcohols
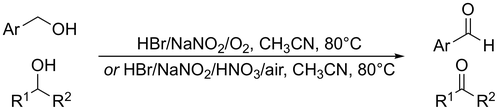
Muhammet Uyanik, Ryota Fukatsu, Kazuaki Ishihara*
Chem. Asian J. 2010, 5, 456–460.
- Rank in Most Accessed Articles for monthly (Feb. 2010) and for the year (2009-2010).
- Highlighted in Journal of Synthetic Organic Chemistry, Japan 2010, 68(8), p873.
7. Hypervalent Iodine-Catalyzed Oxylactonization of Ketocarboxylic Acids to Ketolactones
Muhammet Uyanik, Takeshi Yasui, Kazuaki Ishihara*
Bioorg. Med. Chem. Lett. 2009, 19, 3848–3851.
DOI: 10.1016/j.bmcl.2009.03.148
- Special Issue: the 2009 Tetrahedron Young Investigator Award in Bioorgaic and Medicinal Chemistry in honor of its recipient Prof. C. F. Barbas.
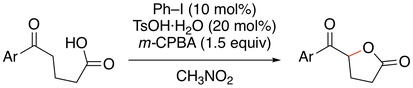
6. IBS-Catalyzed Oxidative Rearrangement of Tertiary Allylic Alcohols to Enones with Oxone
Muhammet Uyanik, Ryota Fukatsu, Kazuaki Ishihara*
Org. Lett. 2009, 11, 3470–3473.

5. Hypervalent Iodine Mediated Oxidation of Alcohols
Muhammet Uyanik, Kazuaki Ishihara*
Chem. Commun. 2009, 45, 2086–2099. (Feature Article)
- Rank in ChemComm Most Cited Feature Articles 2009 Top 10.

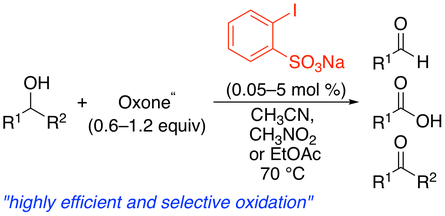
4. 2-Iodoxybenzenesulfonic Acid as an Extremely Active Catalyst for the Selective Oxidation of Alcohols to Aldehydes, Ketones, Carboxylic Acids, and Enones with Oxone
Muhammet Uyanik, Matsujiro Akakura, Kazuaki Ishihara*
J. Am. Chem. Soc. 2009, 131, 251–262.
- Rank in JACS Top 20 Most Read Articles (12 Months).
- Highlighted in Japanese Newspaper (Chunichi Shimbun).
- Highlighted in Gendai Kagaku (by Sato Kentaro, 2009, March (No.456), pp16-19).
- Featured in Org. Chem. Highlights: Best Synthetic Methods: Oxidation.
- Highlighted in Specialty Chemicals Magazine (by Osamu Yamada, 2011, 31(1), pp18-20.
- Nissan Chemical Industries, Ltd. has concluded an exclusive license for the IBS (Press Release: Kagaku Kogyo Nippo).
- pre-IBS and pre-MIBS are commercially available from Junsei Chemical, Ltd., Sigma-Aldrich, TCI Co., Ltd. and Wako Pure Chemical Industries, Ltd.
3. Catalytic Diastereoselective Polycyclization of Homo(polyprenyl)arene Analogues Bearing Terminal Siloxyvinyl Groups
Muhammet Uyanik, Kazuaki Ishihara,* Hisashi Yamamoto*
Org. Lett. 2006, 8, 5649–5652.

2. Biomimetic Synthesis of Acid-Sensitive (−)- and (+)-Caparrapi Oxides, (−)- and (+)-8-Epicaparrapi Oxides, and (+)-Dysifragin Induced by Artificial
Cyclases
Muhammet Uyanik, Kazuaki Ishihara,* Hisashi Yamamoto*
Bioorg. Med. Chem. 2005, 13, 5055–5065.
DOI: 10.1016/j.bmc.2005.04.029
- Special Issue: Chemistry and Biology of Natural Products-Tetrahedron Prize for Creativity in Organic Chemistry 2004: K. Nakanishi.

1. Biomimetic Synthesis of Acid-Sensitive (−)-Caparrapi Oxide and (+)-8-Epicaparrapi Oxide Induced by Artificial Cyclases
Muhammet Uyanik, Hideaki Ishibashi, Kazuaki Ishihara,* Hisashi Yamamoto*
Org. Lett. 2005, 7, 1601–1604.

Invited Lectures
-
2019 Nagoya University GTR Retreat Seminar, Suzuka Circuit, Mie, Japan
-
2018 52nd GIC Seminar, AIST Tohoku Center, Sendai, Japan
-
2018 3rd Workshop on Organic Chemistry, Kyoto University, Japan
- 2017 M&M SYNTECH UNIT Int. Meeting, Nagoya University, Japan
- 2017 HALCHEM VIII, Inuyama, Japan
- 2017 Award Lecture, The 97th Annual Meeting of CSJ, Keio University, Yokohama, Japan
- 2017 Organic Seminar, Okayama University, Okayama, Japan
- 2016 2nd Joint Workshop on WCCU and SMA, Chiba University, Chiba, Japan
- 2016 National Institutes of Natural Sciences, IMS, Okazaki, Japan
- 2015 The University of Tokyo, Graduate School of Pharmaceutical Sciences, Japan
- 2015 Anatolian Conference on Synthetic Organic Chemistry (ACSOC), Antalya, Turkey
- 2014 MEXT Canada/Japan Symposium (pre-ISHC), University of Ottawa, Canada
- 2014 7th Symposium of Molecular Activation, Hokkaido University, Sapporo, Japan
- 2014 JST A-STEP Matchmaking Events, Sendai Kokusai Center, Sendai, Japan
- 2013 The 4th International Symposium on Molecular Activation, Kagoshima Japan
- 2013 93nd Annual Meeting of CSJ, Ritsumeikan University, Shiga, Japan
- 2012 Tokyo University of Sciences, Faculty of Pharmaceutical Sciences, Chiba, Japan
- 2012 Award Lecture, 92nd Annual Meeting of CSJ, Keio University, Tokyo, Japan
- 2012 The 1st Nagoya Symposium on Green Synthesis & Catalysis, Nagoya Univ., Japan
- 2011 25th National Chemistry Congress of Turkish Chemical Society, Erzurum, Turkey
- 2010 Award Lecture, Incentive Award for Young Scientist, Shinshu Univ., Nagano, Japan
Patents
1. カルボニル化合物の製造方法 (Method for producing carbonyl compound)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University)
JP Patent Pub. No. JP2008-247872(A), 2008.10.16 (Appl. No. JP2007-094377, 2007.3.30).
2. カルボニル化合物の製造方法およびカルボニル化合物の製造に用いる酸化促進剤 (Method for producing carbonyl compound and pro-oxidant used for production of carbonyl compound)
Kazuaki Ishihara, Muhammet Uyanik, Yukihiro Isogai, Suguru Ohara (National University Corporation Nagoya University)
JP Patent Reg. No. JP-4590514(B2), 2010.9.24 (Pub. No. JP2010-100636(A), 2010.5.6; Appl. No. JP2009-284483, 2009.12.15; Priority No. JP2007-0226843, 2007.8.31).
PCT Pub. No. WO2009/028676(A1), 2009.3.5 (Appl. No. PCT/JP2008/065567, 2008.8.29).
EP Patent Reg. No. EP-2085373(B1), 2013.1.16 (Pub. No. EP-2085373(A1), 2009.8.5; Appl. No. EP-08828094, 2008.8.29).
US Patent Reg. No. US-8394995(B2), 2013.3.12 (Pub. No. US-2010/0041917(A1), 2010.2.18; Appl. No. US-12/441194, 2008.8.29).
CN Patent Reg. No. CN-101541725(B), 2013.8.28 (Pub. No. CN-101541725(A), 2009.9.23; Appl. No. CN-200880000718, 2008.8.29).
3. 光学活性な環状エーテル化合物の製法及びそれに用いる触媒 (Method for producing optically active cyclic ether compound and catalyst used for the same)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation, Nagoya University)
JP Patent Reg. No. JP-5544596(B2), 2014.5.23 (Pub. No. JP2011-57648, 2011.3.24; Appl. No. JP2009-211609, 2009.9.14).
4. ヨードベンゼン誘導体及びそれを用いた光学活性スピロラクトン化合物の製法 (Iodobenzene derivative and process for preparation of optically active spirolactone compound using same)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University)
JP Patent Reg. No. JP-5747332(B2), 2015.5.22 (Pub. No. WO2011/052388(A1), 2011.5.5; Appl. No. JP2011-538342, 2010.10.14; Priority No. JP2009-245275, 2009.10.26).
PCT Pub. No. WO2011/052388(A1), 2011.5.5 (Appl. No. PCT/JP2010-068031, 2010.10.14).
5. α-アシロキシカルボニル化合物の製法及び新規なα-アシロキシルカルボニル化合物 (Method for producing α-acyloxycarbonyl compound and novel α-acyloxycarbonyl compound)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University; Mitsubishi Rayon Co., Ltd.)
JP Patent Reg. No. JP-5770160(B2), 2015.7.3 (Pub. No. WO2011/108696(A1), 2011.9.9; Appl. No. JP2012-503280, 2011.3.4; Priority No. JP2010-049003, 2010.3.5).
PCT Pub. No. WO2011/108696(A1), 2011.9.9 (Appl. No. PCT/JP2011/055043, 2011.3.4).
EP Patent Reg. No. EP-2543658(B1), 2015.5.6 (Pub. No. EP-2543658(A1), 2013.1.9; Appl. No. EP-11750805, 2011.3.4).
US Patent Reg. No. US-8680322(B2), 2014.3.25 (Pub. No. US-2012/0323014(A1), 2012.12.20; Appl. No. US-13/581734, 2011.3.4).
CN Patent Reg. No. CN-102834368(B), 2015.4.1 (Pub. No. CN-102834368(A), 2012.12.19; Appl. No. CN-201180012595, 2011.3.4).
CN Patent Reg. No. CN-103864608(B), 2016.3.16 (Pub. No. CN-103864608(A), 2014.6.8; Appl. No. CN-201410078904, 2011.3.4).
TW Patent Reg. No. TW-I516473(B), 2016.1.11 (Pub. No. TW-201139360(A1), 2011.11.16; Appl. No. TW-100107298, 2011.3.4).
KR Patent Reg. No. KR-101792298(B1), 2017.10.15 (Pub. No. KR-20130004282(A), 2013.1.9; Appl. No. KR-20127023122, 2011.3.4).
6. エステルの製法 (Method for producing esters)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University)
JP Patent Reg. No. JP-5920889(B2), 2016.4.22 (Pub. No. WO2012/060185(A1), 2012.5.10; Appl. No. JP2012-541793, 2011.10.11; Priority No. JP2010-245944, 2010.11.2).
PCT Pub. No. WO2012/060185(A1), 2012.5.10 (Appl. No. PCT/JP2011/073340, 2011.10.11).
EP Patent Reg. No. EP-2636665(B1), 2016.6.22 (Pub. No. EP-2636665(A1), 2013.9.11; Appl. No. EP-11837836, 2011.10.11).
US Patent Reg. No. US-8853426(B2), 2014.10.7 (Pub. No. US-2013/0217898(A1), 2013.8.22; Appl. No. US-13/881544, 2011.10.11).
7. 窒素原子又は酸素原子を含む環構造を有する芳香族化合物の製造方法 (Method for producing aromatic compound having ring structure that includes nitrogen atom or oxygen atom)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University)
JP Patent Reg. No. JP-5929767(B2), 2016.5.13 (Pub. No. WO2012/111449(A1), 2012.8.23; Appl. No. JP2012-557880, 2012.2.2; Priority No. JP2011-033808, 2011.2.18).
PCT Pub. No. WO2012/111449(A1), 2012.8.23 (Appl. No. PCT/JP2012/052424, 2012.2.2).
US Patent Reg. No. US-9018394(B2), 2015.4.28 (Pub. No. US-2013/0338371(A1), 2013.12.19; Appl. No. US-13/982424, 2012.2.2).
8. ヨードアレーン誘導体、それを用いた光学活性スピロラクトン化合物の製法及び光学活性な環化付加体の製法 (Iodoarene derivative, method for producing optically active spirolactone compound using same, and method for producing optically active cyclization adduct)
Kazuaki Ishihara, Muhammet Uyanik, Takeshi Yasui (National University Corporation Nagoya University)
JP Patent Reg. No. JP-5688647(B2), 2015.2.6 (Pub. No. WO2012/121248(A1), 2012.9.13; Appl. No. JP2013-503554, 2012.3.6; Priority No. JP2011-052572, 2011.3.10).
PCT Pub. No. WO2012/121248(A1), 2012.9.13 (Appl. No. PCT/JP2012/055679, 2012.3.6).
EP Patent Pub. No. EP-2684863(A1), 2014.1.15 (Appl. No. EP-12754890, 2012.3.6).
US Patent Reg. No. US-9000216(B2), 2015.4.7 (Pub. No. US-2013/0338364(A1), 2013.12.19; Appl. No. US-14/002574, 2012.3.6).
CN Patent Pub. No. CN-103415501(A), 2013.11.27 (Appl. No. CN-201280012292, 2012.3.6).
9. 光学活性2-アシルクロマン化合物の製法およびそれに用いる触媒前駆体 (Method for producing optically active 2-acylchroman compound and precatalyst used for the same)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University)
JP Patent Reg. No. JP-6004330(B2), 2016.9.16 (Pub. No. JP2014-51437(A), 2014.3.20; Appl. No. JP2012-194893, 2012.9.5).
10. ビアリール化合物の製造方法 (Production method for biaryl compound)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University)
JP Patent Pub. No. WO2013/121873(A1), 2013.8.22 (Appl. No. JP2014-500157, 2013.1.29; Priority No. JP2012-031223, 2012.2.16).
PCT Pub. No. WO2013/121873(A1), 2013.8.22 (Appl. No. PCT/JP2013/051838, 2013.1.29).
11. 含窒素環化合物の製造方法 (Production method for nitrogen-containing cyclic compound, and nitrogen-containing cyclic compound)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University)
JP Patent Pub. No. WO2013/121879(A1), 2013.8.22 (Appl. No. JP2014-500160, 2013.1.30; Priority No. JP2012-031224, 2012.2.16).
PCT Pub. No. WO2013/121879(A1), 2013.8.22 (Appl. No. PCT/JP2013/051983, 2013.1.30).
12. 窒素原子を含む環構造を有する光学活性な芳香族化合物の製法 (Method for producing optically active aromatic compounds having ring structure that includes nitrogen atom)
Kazuaki Ishihara, Muhammet Uyanik, Daisuke Suzuki (National University Corporation Nagoya University)
JP Patent Reg. No. JP-6115946(B2), 2017.3.31 (Pub. No. JP2014-240365(A), 2014.12.25; Appl. No. JP2013-123423, 2013.6.12).
13. アリールヒドロキノン類の製法及びアリールキノン類の製法 (Method for producing aryl hydroquinone derivative and aryl quinone derivative)
Kazuaki Ishihara, Muhammet Uyanik (National University Corporation Nagoya University)
JP Patent Pub. No. JP2015-168660, 2015.9.28 (Appl. No. JP2014-046259, 2014.3.10).
14. 新規なアルキルカルボニルラクトン化合物及びその製造方法 (New alkyl carbonyl lactone compounds and method for producing thereof)
Kazuaki Ishihara, Muhammet Uyanik, Kikuo Furukawa, Hiroyasu Tanaka (National University Corporation Nagoya University; Mitsubishi Gas Co. Ltd.)
JP Patent Reg. No. JP-6404666(B2), 2018.9.21 (Pub. No. JP2016-74614(A), 2016.5.12; Appl. No. JP2014-204756, 2014.10.3).
External Grants
2007 JST Seeds Excavation Examination (08-477)
2010-2012 JSPS Grant-in-Aid for Young Scientists_B (22750087)
2010 JST A-STEP (AS221Z02039D)
2011-2012 JST A-STEP (AS232Z02306D)
2012-2013 JST A-STEP (AS242Z00126M)
2012-2013 JST A-STEP, East Japan Earthquake Revival Promotion (241FT0209)
2013 JSPS Challenging Exploratory Research (25620078)
2013-2014 JSPS Scientific Research on Innovative Areas: Molecular Activation (25105722)
2015-2017 JSPS Grant-in-Aid for Young Scientists_A (15H05484)
2018-2020 JSPS Grant-in-Aid for Scientific Research_B (18H01973)
2021-2023 JSPS Grant-in-Aid for Scientific Research_B (21H01932)
Products
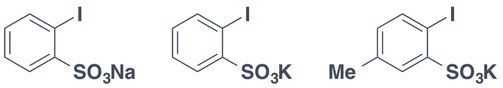
Sodium 2-iodobenzenesulfonate (Pre-IBS) (CAS: 62973-39-7)
Junsei Chemical Ltd.: 80517-1615 (1g).
Potassium 2-iodobenzenesulfonate (Pre-IBS) (CAS: 62973-69-7)
Junsei Chemical Ltd.: 80516-1615 (1g).
Potassium 2-iodo-5-methylbenzenesulfonate (Pre-MIBS)(CAS: 1093215-92-9)
Sigma-Aldrich: 720011-(1g, 5g, 10g)
Junsei Chemical Ltd.: 80545-1615 (1g).
FUJIFILM Wako Pure Chem. Ind., Ltd.: 167-25741 (100mg)163-25743 (1g), 161-25744 (5g).
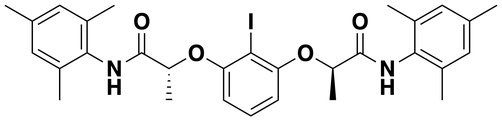
(2R,2'R)-2,2'-(2-Iodo-1,3-phenylene)bis(oxy)bis(N-mesitylpropanamide)
(CAS: 1226896-38-3)
FUJIFILM Wako Pure Chem. Ind., Ltd.: 095-06051 (250 mg), 091-06053 (1 g).
TCI Co. Ltd.: I0807 (200 mg).

N,N'-[(2S,2'S)-[(2-Iodo-1,3-phenylene)bis(oxy)]bis(propane-2,1-diyl)]bis(mesitylamide)
(CAS: 1399008-27-5 )
TCI Co. Ltd.: I1121 (200 mg).