論文 2019

▼TCIメールへの寄稿論文
「デザイナーC2対称ジアミド型キラルヨードアレーン触媒」
ウヤヌク ムハメット、石原 一彰
TCIメール 2019, No. 182, 2–16.
"Designer C2-symmetric Chiral Diamide-type Organoiodine Catalysts"
Muhammet Uyanik and Kazuaki Ishihara
TCIMAIL, 2019, No. 182, 2–14.
▼"High-Performance Ammonium Hypoiodite/Oxone Catalysis for Enantioselective Oxidative Dearomatization of Arenols"
Muhammet Uyanik, Takehiro Kato, Naoto Sahara, Outa Katade, Kazuaki
Ishihara*
ACS Catal. 2019, 9(12), 11619–11626.
Publication Date: November 14,
2019
https://doi.org/10.1021/acscatal.9b04322

▼An enantioselective oxidative coupling reaction of 2-naphthol
derivatives catalyzed by chiral diphosphine oxide–iron(ii) complexes
Takahiro Horibe, Keita Nakagawa, Takashi Hazeyama, Kazuki Takeda and Kazuaki Ishihara*
Chem. Commun. 2019, 55(91), 13677–16680.
https://doi.org/10.1039/C9CC07834G

▼[解説]
"デザイン型Brønsted酸触媒を用いるエナンチオ選択的アザ-Friedel–Crafts反応"
波多野学, 石原一彰, 触媒 2019, 61(5),298–304.
https://www.shokubai.org/jnl/
▼"ホウ素Lewis酸—キラルリン酸複合触媒を用いるマルチ選択的[2+2]/[4+2]付加環化反応"
波多野学,石原一彰
化学工業 2019, 70(9),
634–642. 発行日:2019年9月1日
http://www.kako-sha.co.jp/newvol.htm#kagaku
▼巻頭言「ヨウ素を知るには隣のハロゲン・カルコゲンに着目を」
石原一彰
ヨウ素学会SIS Letters 2019, No. 20, 1(2019年5月第20号)
ヨウ素学会でヨウ素の科学をフォーカスするのは当然のことであるが、ヨウ素はハロゲン族(フッ素、塩素、臭素、ヨウ素、アスタチン、テネシン)の一元素であり、ヨウ素の特徴を引き出すには他のハロゲン元素との比較が不可欠である。研究者であれば、周期表におけるヨウ素の一つ上に位置する臭素に特に着目したい。一方、ヨウ素の下に位置するアスタチンは強い放射能と短い半減期のため、研究用以外に用途はない。
私は有機反応の触媒や反応剤の開発に関する研究をしているが、それらの鍵元素としてヨウ素に強い関心を持っている。ヨウ素を遷移金属や重金属の代替元素として利用して触媒や反応剤が開発できれば環境低負荷有機合成技術の向上に繋がるからである。実際、研究してみると有機反応の基質や反応様式の違いによってヨウ素を利用した方がよい場合と臭素を利用した方がよい場合があることに気づかされる。
また、ハロゲン族の左隣にはカルコゲン族があり、ヨウ素の左隣はテルルである。近年、ハロゲン結合に注目が集まっているが、昨年のハロゲン結合国際会議では、カルコゲン結合についても議論されており目が離せない。
ヨウ素の化学的特性を深く知り、それを活かすためには、他のハロゲン・カルコゲン元素についても同様に知ることが必要であり、ヨウ素だけにフォーカスしていると井の中の蛙になる恐れがある。
▼塩化鉄(III)を用いた芳香族ラジカルカチオンの単離と反応性
堀部貴大、大村修平、石原一彰
ケミカルエンジニヤリング 2019, 64(5), 297–304. (発行日:2019年5月1日)
▼Highly Active Chiral Dilithium(I) Binaphthyldisulfonate Catalysts for Enantio- and Chemoselective Strecker-Type Reactions
Manabu Hatano, Kosuke Nishio, Takuya Mochizuki, Keisuke Nishikawa, Kazuaki Ishihara*
ACS Catal. 2019, 9(xxx), 8178-8186.
Publication Date:July 30, 2019
DOI: 10.1021/acscatal.9b02739
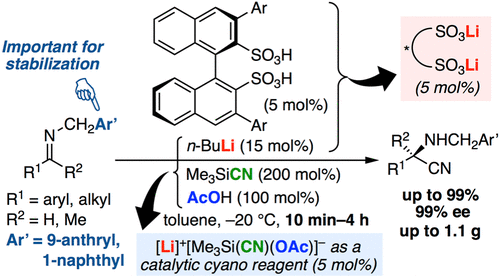
An enantioselective Strecker-type reaction of aldimines and ketimines was developed by using a chiral dilithium(I) binaphthyldisulfonate as a chiral acid–base cooperative catalyst. The present catalytic system features an extremely short reaction time (10 min to 4 h), unlike conventional catalytic systems. Along with the design of stronger chiral Li(I) Lewis acid catalysts, a highly reactive pentacoordinate silicate generated in situ could promote the reactions. In particular, instead of unstable N-Bn Strecker products, more stable N-CH2(9-anthryl) and N-CH2(1-naphthyl) Strecker products could be obtained in high yields with high enantioselectivities. By a switch of the present and previous catalyst systems, chemoselective cyanation to a ketoaldimine could be performed, respectively. Moreover, mechanistic investigations provided useful information regarding the active catalysts, catalytic cycles, and possible transition states.
▼Tris(pentafluoropenyl)borane-Assisted Chiral Phosphoric Acid Catalysts for Enantioselective Inverse-Electron-Demand Hetero-Diels–Alder Reaction of α,β‐Substituted Acroleins
Manabu Hatano, Tatsuhiro Sakamoto, Takuya Mochizuki, Kazuaki Ishihara*
Asian J. Org. Chem. 2019, 8(7), 1061-1066.
First Published: 06 March 2019
https://doi.org/10.1002/ajoc.201900104

In the enantioselective inverse‐electron‐demand hetero‐Diels‐Alder reaction, simple α,β‐unsaturated aldehydes (i. e., acroleins) are still challenging substrates, unlike α,β‐unsaturated carbonyl compounds containing electron‐withdrawing groups. In the present study, the reaction of α‐aryl‐β‐alkyl‐substituted acroleins with ethyl vinyl sulfide was developed with the use of bulky chiral supramolecular Brønsted acid catalysts, such as tris(pentafluorophenyl)borane‐assisted chiral phosphoric acid catalysts. As a result, cis‐cycloadducts as optically active 3,4‐dihydro‐2H‐pyrans were exclusively obtained in high yields with high enantioselectivities via the favored endo orbital approach. An obtained optically active cis‐isomer could be transformed into the corresponding trans‐isomer without a loss of enantiopurity by O,S‐acetal epimerization. Moreover, transformations to synthetically useful optically active epoxide and δ‐valerolactone are also demonstrated.
▼Front Cover: "Tris(pentafluorophenyl)borane‐Assisted Chiral Phosphoric Acid Catalysts for Enantioselective Inverse‐Electron‐Demand Hetero‐Diels‐Alder Reaction of α,β‐Substituted Acroleins" (Asian J. Org. Chem. 2019, 8(7), 1061 Special Issue: Heterocyclic Chemistry)
First published: 26 June 2019
DOI: 10.1002/ajoc.201900184

▼Structure and Reactivity of Aromatic Radical Cations Generated by FeCl3
J. Am. Chem. Soc. 2019, 141(5), 1877–1881.
Takahiro Horibe, Shuhei Ohmura, and Kazuaki Ishihara*
DOI: 10.1021/jacs.8b12827
Publication Date (Web): January 24, 2019

This paper describes the isolation and characterization of an aromatic radical cation generated by FeCl3. X-ray crystallographic analysis and kinetic studies reveal the mechanism of the generation of aromatic radical cation. In the solid state, a tight ion-pair of a radical cation with FeCl4– is observed. Leveraging the efficient generation of the radical cation–FeCl4– ion pair, we explore a radical cation-induced cycloaddition of trans-anethole initiated by catalytic amount of FeCl3. Both [4+2] cycloaddition and [2+2] cycloaddition with a broad substrate scope are also described. Moreover, a 100 g-scale reaction is demonstrated with the use of 1 mol % of FeCl3 as a simple and a highly active initiator.
▼Ammonium Hypoiodite-catalyzed Oxidative Dearomatizative Azidation of Arenols
Muhammet Uyanik, Kohei Nishioka, and Kazuaki Ishihara*
Chem. Lett. 2019, 48(4), 353–356.
https://doi.org/10.1246/cl.181036
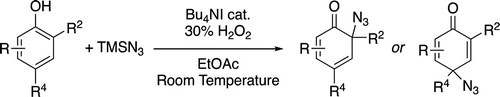
The first transition metal-free catalytic oxidative dearomatizative azidation of arenols has been developed using hypoiodite catalysis with aqueous hydrogen peroxide and trimethylsilyl azide as an oxidant and azide source, respectively, under mild conditions. The corresponding quaternary azides can be obtained in high to excellent yields.
▼Enantioselective [1,3] O-to-C Rearrangement: Dearomatization of Alkyl 2-Allyloxy/benzyloxy-1/3-naphthoates Catalyzed by a Chiral π–Cu(II)
Complex
Lu Yao and Kazuaki Ishihara*
Chem. Sci. 2019, 10(8), 2259–2263.
The article was first published on 10 Jan 2019
http://dx.doi.org/10.1039/C8SC05601C

An unprecedented catalytic asymmetric [1,3] O-to-C rearrangement of alkyl 2-allyloxy/benzyloxy-1/3-naphthoates was realized under the catalysis of a chiral π–Cu(II) complex (1–10 mol%). This dearomatization strategy provides facile access to highly functionalized β-naphthalenone derivatives bearing an all-carbon quaternary stereogenic center in high yield with excellent enantioselectivity. The π–cation interaction between the aromatic substituent of the ligand and the Cu(II) center was proved by X-ray diffraction analysis and shown to be crucial for enantioselective control. Further preliminary mechanistic studies suggest that this intramolecular reaction proceeds through a contact ion pair intermediate.
▼Inside Front Cover Picture (Chem. Sci. 2019,
10(8))

▼This paper was ranked 1st in the list of HOT Chemical Science Articles for January (28 February, 2019)
▼Very Important Paper
Regioselective Oxidative Chlorination of Arenols Using NaCl and
Oxone
Eur. J. Org. Chem. 2019, (1), 27–31.

A practical and efficient chlorination of naphthols and phenols was developed using transient chlorinating species generated in situ from inexpensive sodium chloride and Oxone as a Cl source and oxidant, respectively, under mild conditions.
▼Front Cover Picture (Eur. J. Org. Chem. 2019, No.
1.)
https://onlinelibrary.wiley.com/doi/epdf/10.1002/ejoc.201801769