論文 2018

▼[Author Profile] Kazuaki Ishihara
Eur. J. Org. Chem. 2019, (1), 6.
First published: 12 October 2018
https://doi.org/10.1002/ejoc.201801455
▼Chiral Supramolecular U-Shaped Catalysts Induce the Multiselective Diels–Alder Reaction of Propargyl Aldehyde
Publication Date (Web): November 7, 2018

The Diels–Alder reaction, which is a traditional [4 + 2] cycloaddition with two carbon–carbon bond formations, is one of the most powerful tools to synthesize versatile and unique six-membered rings. We show that chiral supramolecular U-shaped boron Lewis acid catalysts promote the unprecedented multiselective Diels–Alder reaction of propargyl aldehyde with cyclic dienes. Independent from the substrate control, enantio-, endo/exo-, π-facial-, regio-, site-, and substrate-selectivities could be controlled by the present U-shaped catalysts. The obtained reaction products could access the concise synthesis of chiral diene ligands and a key intermediate of (+)-sarkomycin. The results presented here might partially contribute to the development of artificial enzyme-like supramolecular catalysts for multiselective reactions, which will be able to target organic compounds that have thus far eluded synthesis.

▼Oxidation of alcohols and
amines
Muhammet Uyanik and Kazuaki Ishihara
In “Patai’s Chemistry of Functional Groups,” edited by Ilan Marek, Berit Olofsson, Zvi Rappoport
pp. 261–306. John Wiley & Sons, Ltd: Chichester, UK.
1032 pages
DOI: 10.1002/9780470682531.pat0945.
Published online 17 SEP 2018.
ISBN: 978-1-119-35230-3
April 2019,
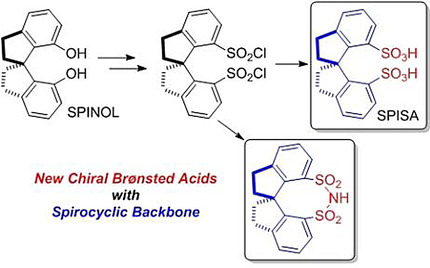
▼Synthesis of 1,1′‐Spirobiindane‐7,7′‐Disulfonic Acid and Disulfonimide: Application for Catalytic Asymmetric Aminalization
Chem. Asian J. 2018, 13(17), 2378-2381.[Special Issue: Homogeneous Catalysis from Young Investigators in Asia]
The article was first published on 12 April 2018
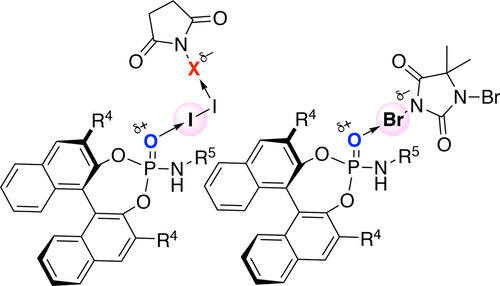
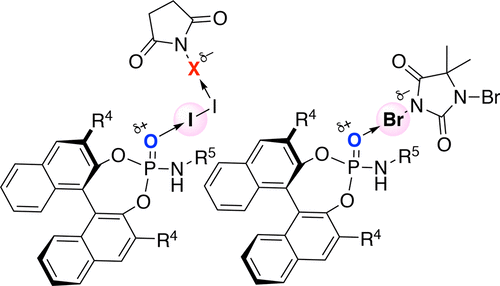
▼Enantioselective Halo-oxy- and Halo-azacyclizations Induced by Chiral Amidophosphate Catalysts and Halo-Lewis Acids
Yanhui Lu, Hidefumi Nakatsuji, Yukimasa Okumura, Lu Yao, and Kazuaki Ishihara*
J. Am. Chem. Soc. 2018, 140(19), 6039-6043.
DOI: 10.1021/jacs.8b02607
Publication Date (Web): April 30, 2018
https://pubs.acs.org/doi/pdf/10.1021/jacs.8b02607
Ranked in the journal’s Top 20 most downloaded articles for the previous 30 days (14th June, 2018).
https://pubs.acs.org/action/showMostReadArticles?topArticlesType=month&journalCode=jacsat
"Chiral Amidophosphate-Induced Halooxy- and Haloazacyclizations"
Contributors: Benjamin List and Mathias Turberg
Synfacts 2018, 14(8), 864.
DOI: 10.1055/s-0037-1609894
▼ホウ酸・ボロン酸触媒を用いるアミド縮合反応の開発と工業利用
発行日:2018年3月30日
https://www.cmcbooks.co.jp/products/detail.php?product_id=5419
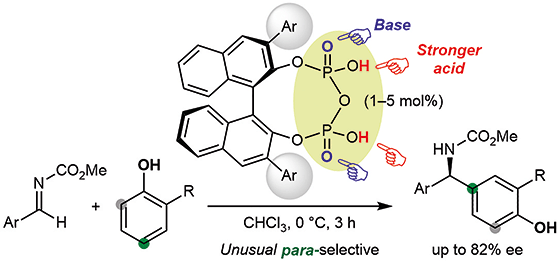
▼Chiral Pyrophosphoric Acid Catalysts for the para-Selective and Eanantioselective Aza-Friedel–Crafts Reaction of Phenols
The article was first published on 22 August 2018
Synthesis 2018, 50(23), 4577-4590.
Chiral BINOL-derived pyrophosphoric acid catalysts were developed and used for the regio- and enantioselective aza-Friedel–Crafts reaction of phenols with aldimines. ortho/para-Directing phenols could react at the para-position selectively with moderate to good enantioselectivities. Moreover, the gram-scale transformation of a product into the key intermediate for the antifungal agent (R)-bifonazole was demonstrated.

▼[北大 松永研との共同研究成果]
"Pentamethylcyclopentadienyl rhodium(III)–chiral disulfonate hybrid catalysis for enantioselective C–H bond functionalization"
Shun Satake, Takumaru Kurihara, Keisuke Nishikawa, Takuya Mochizuki, Manabu Hatano, Kazuaki Ishihara, Tatsuhiko Yoshino* & Shigeki Matsunaga*
Nat. Catal. 2018, 1(8), 585–591. Published: 23 July 2018
DOI: 10.1038/s41929-018-0106-5
https://www.nature.com/articles/s41929-018-0106-5
プレスリリース
http://www.nagoya-u.ac.jp/.../upload.../20180724_engg_1.pdf
日刊工業新聞(2018年8月2日25面)
見出し「1工程で鏡像異性体合成 北大など」
https://www.nikkan.co.jp/articles/view/00483484?isReadConfirmed=true
国立環境研究所ニュース(2018年7月24日)
見出し「北大と名大、医薬品合成における環境負荷低減に役立つハイブリッド触媒の作製に成功」
http://tenbou.nies.go.jp/news/jnews/detail.php?i=24703
Press Release (August 9, 2018)
Hybrid catalyst with high enantiomer selectivity
▼ホウ素触媒でカルボン酸を自在に変換する! アシロキシボラン中間体を鍵とするエナンチオ選択的変換反応
堀部貴大、石原一彰
化学 2018, 73(8), 25–28.
▼私の自慢「根拠のないプライドを支えに、アカデミアの世界へ ポスドクでやり残したもの」
石原一彰
化学と工業 2018, 71(7), 598–601.
http://www.chemistry.or.jp/journal/ci1807.pdf
▼Enantioselective Aza-Friedel–Crafts Reaction of Furan with α-Ketimino Esters Induced by a Conjugated Double Hydrogen Bond Network of Chiral Bis(phosphoric Acid) Catalysts
The article was first published on 25 Jun 2018
Chem. Sci. 2018, 9(30), 6361-6367.
DOI: 10.1039/C8SC02290A
Chiral C2- and C1-symmetric BINOL-derived bis(phosphoric acid) catalysts, which have OP(=O)(OH)2/OP(=O)(OH)(OR) moieties at the 2,2’-positions, were developed and used for the enantioselective aza-Friedel–Crafts reaction of 2-methoxyfuran with α-ketimino esters for the first time. The intramolecular conjugated double hydrogen bond network is a key to increasing the Brønsted acidity and preventing deactivation of the catalysts. Highly functionalized α-amino acid derivatives with a chiral quaternary carbon center could be transformed into versatile optically active N- and O-heterocycles and an α-aryl-substituted serine.
Selected as a Back Cover Picture
Chem. Sci. 2018, 9(30), 6453-6454.
DOI: 10.1039/C8SC90158A

▼Asymmetric Total Synthesis of (−)-Maldoxin, a Common Biosynthetic Ancestor of the Chloropupukeananin Family
DOI: 10.1021/acs.orglett.8b01502

▼Thiourea–I2 as Lewis Base–Lewis
Acid Cooperative Catalysts for Iodochlorination of Alkene with In Situ-Generated I–Cl
Takahiro Horibe , Yasutaka Tsuji, and Kazuaki Ishihara*
ACS Catal. 2018, 8(7), 6362–6366
DOI: 10.1021/acscatal.8b01565
Publication Date (Web): June 7, 2018
https://pubs.acs.org/doi/10.1021/acscatal.8b01565
"Cooperative Lewis Base–Lewis Acid Catalysis for Iodochlorination of alkenes"
Contributors: Benjamin List, David Días-Oviedo
Synfacts 2018; 14(09): 0976
DOI: 10.1055/s-0037-1610598
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0037-1610598

▼Enantioselective Halo-oxy- and Halo-azacyclizations Induced by Chiral Amidophosphate Catalysts and Halo-Lewis Acids
Yanhui Lu, Hidefumi Nakatsuji, Yukimasa Okumura, Lu Yao, and Kazuaki Ishihara*
J. Am. Chem. Soc. 2018, 140(19), 6039-6043.
DOI: 10.1021/jacs.8b02607
Publication Date (Web): April 30, 2018
https://pubs.acs.org/doi/pdf/10.1021/jacs.8b02607
▼ortho-Substituent Effect on 2,4-Bis(trifluoromethyl)phenylboronic Acid-Catalyzed Dehydrative Condensation between Carboxylic Acids
and Amines
Ke Wang, Yanhui Lu and Kazuaki Ishihara*
Chem. Commun. 2018, 54(43), 5410–5413. (Inside Cover Picture)
The article was first published on 24 Apr 2018

▼Chapter 10. α-Oxidation of Carbonyl Compounds
In "Science of Synthesis, Catalytic Oxidation in Organic Synthesis," Volume Editor: Kilian Muñiz, Thieme Chemisty
▼ボロン酸触媒を用いるアミド縮合反応
石原一彰
PETROTECH 2018, 41(1), 16–19.
PETROTECH 〜石油学会情報誌〜
特集ファインケミカルズ合成のための触媒研究最前線
http://www.sekiyu-gakkai.or.jp/…/kank…/petro/petropress.html
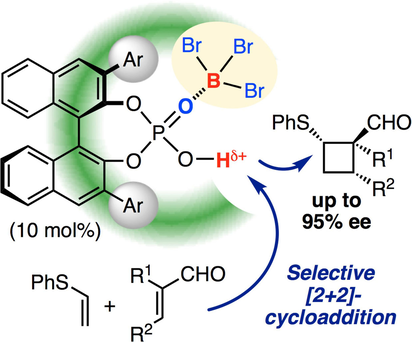
▼Boron Tribromide‐Assisted Chiral Phosphoric Acid Catalysts for Enantioselective [2+2] Cycloaddition
First published: 30 March 2018
https://doi.org/10.1002/asia.201800351
DOI: 10.1002/asia.201800351
Proton challenge: BBr3‐assisted chiral phosphoric acid catalysts were used for the enantioselective [2+2] cycloaddition of vinyl sulfides with acroleins. One of the obtained [2+2] cycloadducts could be readily transformed to a key intermediate for (+)‐frontalin as a pheromone of Asian elephants.
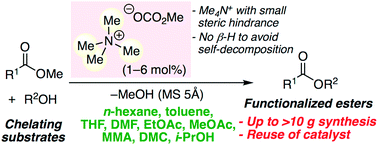
▼Metal-Free Transesterification Catalyzed by Tetramethylammonium Methyl Carbonate
Manabu Hatano, Yuji Tabata, Yurika Yoshida, Kohei Toh, Kenji Yamashita, Yoshihiro Ogura, and Kazuaki Ishihara*
Green Chem. 2018, 20(6), 1193–1198.
Environmentally benign metal-free tetramethylammonium methyl carbonate is effective as a catalyst for the chemoselective, scalable, and reusable transesterification of various esters and alcohols in common organic solvents. In situ-generated highly active species, tetramethylammonium alkoxides, can greatly avoid the self-decomposition at ≤110 °C, and reusable. In particular, chelating substrates, such as amino alcohols, diols, triols, sugar derivatives, alkaloids, α-amino acid esters, etc., which deactivate conventional metal salt catalysts, can be used. A 100 gram scale biodiesel production was also demonstrated.
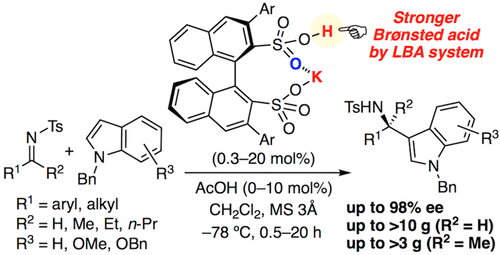
▼Enantioselective Aza-Friedel-Crafts Reaction of Indoles with Ketimines Catalyzed by Chiral Potassium Binaphthyldisulfonates
The enantioselective aza-Friedel–Crafts reaction of indoles with low-reactive ketimines has been developed in the presence of a chiral monopotassium binaphthyldisulfonate as a strong Brønsted acid catalyst. A broad substrate scope was achieved, and the corresponding 3-indolylmethanamines with a chiral quaternary carbon center were obtained in high yields with high enantioselectivities. The addition of a catalytic amount of acetic acid considerably promoted the reaction, and a gram-scale reaction could be achieved with reduced catalyst loading.