論文 2016

▼Book
【特集】ヨウ素触媒の開発最前線 寄稿「キラル第四級アンモニウム次亜ヨウ素酸塩触媒(Chiral Quaternary Ammonium Hypoiodite Catalysis)」
Muhammet Uyanik, 石原一彰
月刊ファインケミカル, 2016, 45(12), 16–24.
2016年12月15日発行、ISBNコード: 0913-6150
過酸化水素あるいはアルコール過酸化物の存在下, キラルヨウ化第四級アンモニウムからin situで調製される次亜ヨウ素酸塩を触媒活性種に用いるエナンチオ選択的酸化的環化反応を開発した。本酸化システムの詳細な触媒機構を解明することで, 高活性な不斉有機酸化触媒システムの構築に成功し, 多くの生物活性物質に含まれる2,3-ジヒドロベンゾフラン, クロマン, テトラヒドロフラン,
およびスピロラクトン骨格の新規不斉合成を達成した。
▼Book
Our chiral supramolecular catalyst was selected as a book cover picture!
Lewis Acids
Manabu Hatano, Kazuaki Ishihara
In Boron Reagents in Synthesis, Editor(s): Adiel Coca, Volume 1236, Chapter 2, pp 27-66.
DOI: 10.1021/bk-2016-1236.ch002
Abstract: This chapter reviews recent progress on boron(III) Lewis acids. Due to the chemical stability and easy molecular design of boron(III) compounds, boron(III) Lewis acids have been used in organic synthesis for more than 80 years both stoichiometrically and catalytically. In particular, this chapter focuses on the recent application of boron(III) Lewis acid catalysts in asymmetric reactions. Recent advances in supramolecular cooperative catalysts involving chiral boron(III) Lewis acids are also reviewed. Moreover, recent interesting examples of acid or base cooperative boronic acid catalysts and recent synthetically important advances with electron-deficient triarylborane(III) are described.
▼Book
Bifunctional Lewis Base Catalysis with Dual Activation of R–M and C–O (n –> σ*)
Manabu Hatano and Kazuaki Ishihara
In Lewis Base Catalysis in Organic Synthesis, 3 Volumes Set, edited by Edwin Vedejs and Scott E. Denmark, 2016, p. 339–386, Wiley-VCH, August 2016. ISBN: 978-3-527-33618-0
http://as.wiley.com/WileyCDA/WileyTitle/productCd-3527336184.html

▼Regioselective 1,4- and 1,6-Conjugate Additions of
Grignard Reagent-Derived Organozinc(II)ates to Polyconjugated Esters
Manabu Hatano, Mai Mizuno, and Kazuaki Ishihara*
Org. Lett. 2016, 18(18), 4462-4465.
Publication Date (Web): September 7, 2016 (Letter)
DOI:
10.1021/acs.orglett.6b01774
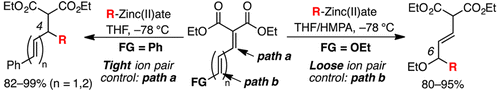
Regioselective synthetic methods were developed for 1,4- and 1,6-conjugate additions of Grignard reagent-derived organozinc(II)ates to malonate-derived polyconjugated esters. By taking advantage of the tight ion-pair control of organozinc(II)ates, it was possible to switch between 1,4- and 1,6-conjugate additions by introducing a terminal ethoxy moiety in the conjugation.
▼キラルビナフチルジスルホン酸(BINSA)を用いる精密分子触媒設計
化学工業 2016, 67(9), 660–667.
【特集】ケミカルバイオロジーの新展開
波多野学、石原一彰

アミノ酸をはじめとする天然物や医薬品の多くは光学活性体で,両鏡像体間で生理活性は大きく異なる.従って,有機合成で自由自在に目的の光学活性化合物を得るためには,触媒的不斉合成法の更なる発展が必要である.なかでも,両鏡像体が安価に入手容易な (R)-または(S)-1,1’-ビナフチル-2,2’-ジオール(BINOL)から誘導されるC2対称なキラルビナフチル化合物は,金属イオンに対するキラル配位子やキラル有機分子触媒として優れた機能を発揮し,多くの不斉触媒反応で用いられている1).本稿では,筆者らが取り組んできたキラルビナフチルジスルホン酸(BINSA)を鍵とするキラル酸・塩基複合触媒2,3)による反応開発について紹介する.
▼Book
Asymmetric Dearomatization Reactions
Chapter 6. Asymmetric Oxidative Dearomatization Reaction
Muhammet Uyanik and Kazuaki Ishihara, pp. 129-151
In "Asymmetric Dearomatization Reactions" edited by Shu-Li You
[ISBN: 978-3-527-33851-1], 424 pages, Wiley-VCH, August 2016.
http://www.wiley.com/WileyCDA/WileyTitle/productCd-3527338519.html

▼New Book
[From Biosynthesis to Total Synthesis: Strategies and Tactics for Natural Products]
Alexandros L. Zografos
584 pages, Wiley, April 2016, ISBN: 978-1-118-75173-2
Chapter 9: Higher Terpenes and Steroids, pp. 296–330
Kazuaki Ishihara
http://as.wiley.com/WileyCDA/WileyTitle/productCd-1118751736,subjectCd-LS30.html

▼Enantioselective Bromocyclization of 2-Gernaylphenols
Induced by Chiral Phosphite–Urea Bifunctional Catalysts
Yasuhiro Sawamura, Yoshihiro Ogura, Hidefumi Nakatsuji, Akira Sakakura* and Kazuaki Ishihara*
Chem. Commun. 2016, 52(36), 6068–6071. DOI: 10.1039/C6CC00229C
http://pubs.rsc.org/en/content/articlelanding/2016/cc/c6cc00229c#!divAbstract

Chiral phosphite–urea bifunctional catalysts have been developed for the enantioselective bromocyclization of 2-geranylphenols with N-bromophthalimide (NBP) for the first time. The chiral triaryl phosphite moiety activates NBP to generate a bromophosphonium ion. On the other hand, the urea moiety interacts with a hydroxyl group of the substrate through hydrogen bonding interactions. Enantioselectivity is effectively induced through two-point attractive interactions between the catalyst and the substrate.

▼リン酸の酸・塩基協奏機能を活用したホウ素Lewis酸-キラルリン酸複合高活性触媒の開発
月刊ファインケミカル 2016年2月号 pp. 24–32.
【特集】不斉合成の進展と最新研究(2016年2月15日発行)
波多野学、石原一彰
http://www.cmcbooks.co.jp/products/detail.php?product_id=5060
Lewis酸複合型キラルBrønsted酸(LBA)の分子触媒設計に立脚し,アキラルなホウ素Lewis酸で活性化されたキラルリン酸触媒を創製した。本複合触媒は従来のリン酸触媒単独では適用困難だったα-置換アクロレイン類の不斉Diels-Alder(DA)反応に有効であり,特にカタランチン系インドールアルカロイド鍵中間体の高選択的合成に成功した。
▼Enantioselective Diels–Alder Reaction Induced by Chiral Supramolecular Lewis Acid Catalysts Based on CN···B and PO···B Coordination Bonds
Manabu Hatano, Kazushi Hayashi, Tatsuhiro Sakamoto, Yuma Makino, Kazuaki Ishihara*
Synlett, 2016, 27, 1061-1067. Web Publication Date: 05 February 2016
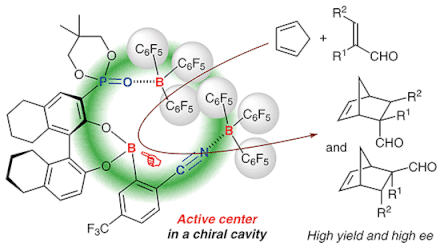
Chiral supramolecular boron Lewis acid catalysts were prepared from chiral 3-phosphoryl-1,1′-bi-2-naphthols, (2-cyanophenyl)boronic acids, and tris(pentafluorophenyl)borane, bound through CN···B and PO···B coordination bonds. In particular, the coordinated tris(pentafluorophenyl)boranes increase the Lewis acidity of the active center in the manner of a Lewis acid assisted Lewis acid catalyst system. A possible cavity in these catalysts was highly suitable for several Diels–Alder probe reactions of acroleins with cyclic or acyclic dienes, which gave the corresponding adducts in good to high yields and high enantioselectivities.
▼Enantioselective Cyanosilylation of Ketones with Lithium(I) Dicyanotrimethylsilicate(IV)
Catalyzed by a Chiral Lithium(I) Phosphoryl Phenoxide
Manabu Hatano, Katsuya Yamakawa, Tomoaki Kawai, Takahiro Horibe, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2016, 55(12), 4021-4025.
Article first published online: 2 FEB 2016.

A highly enantioselective cyanosilylation of ketones was developed by using a chiral lithium(I) phosphoryl phenoxide aqua complex as an acid/base cooperative catalyst. The pentacoordinate silicate generated in situ from Me3SiCN/LiCN acts as an extremely reactive cyano reagent. Described is a 30 gram scale reaction and the synthesis of the key precursor to (+)-13-hydroxyisocyclocelabenzine.
[Cover picture] Enantioselective Cyanosilylation of Ketones with Lithium(I) Dicyanotrimethylsilicate(IV) Catalyzed by a Chiral Lithium(I) Phosphoryl Phenoxide
Hatano, H.; Yamakawa, K.; Kawai, T.; Horibe, T.; K. Ishihara
Angew. Chem. Int. Ed. early view. DOI: 10.1002/anie.201601600
▼Chiral Ammonium Hypoiodite Salt-Catalyzed Enantioselective Oxidative
Cycloetherification to 2-Acyl Tetrahydrofurans
Muhammet Uyanik, Hiroki Hayashi, Hirokazu Iwata, and Kazuaki Ishihara
Chem. Lett. 2016, 45(3), 353–355.

2-Acyl tetrahydrofuran is a fundamental structure in natural products and pharmaceuticals. We achieved a chiral quaternary ammonium hypoiodite salt-catalyzed enantioselective oxidative cycloetherification of δ-hydroxyketone derivatives. The corresponding 2-acyl tetrahydrofurans were obtained in high chemical yield with high enantioselectivity.
▼Book
Chapter 2. Alkali metal (Li, Na, K)-based Catalysts
Manabu Hatano and Kazuaki
Ishihara
pp. 15–48, RSC Green Chemistry No. 38, Sustainable Catalysis: With Non-endangered Metals, Part 1 Edited by Michael North
The Royal Society of Chemistry 2016
http://pubs.rsc.org/en/content/ebook/978-1-78262-056-3#!divbookcontent

▼Structurally Defined Molecular Hypervalent Iodine Catalysts for
Intermolecular Enantioselective Reactions
S. Haubenreisser, T. H. Wöste, C. Martínez, K. Ishihara, K. Muñiz
Angew. Chem. Int. Ed. 2016, 55(1), 413–417. First published: 24 November 2015.
DOI: 10.1002/anie.201507180
http://onlinelibrary.wiley.com/doi/10.1002/anie.201507180/full

Molecular structures of the most prominent chiral non-racemic hypervalent iodine(III) reagents to date have been elucidated for the first time. The formation of a chirally induced supramolecular scaffold based on a selective hydrogen-bonding arrangement provides an explanation for the consistently high asymmetric induction with these reagents. As an exploratory example, their scope as chiral catalysts was extended to the enantioselective dioxygenation of alkenes. A series of terminal styrenes are converted into the corresponding vicinal diacetoxylation products under mild conditions and provide the proof of principle for a truly intermolecular asymmetric alkene oxidation under iodine(I/III) catalysis.
[Cover Picture] Structurally Defined Molecular Hypervalent Iodine Catalysts for Intermolecular Enantioselective Reactions
S. Haubenreisser, T. H. Wöste, C. Martínez, K. Ishihara, K. Muñiz Angew. Chem. Int. Ed. 2016, 55(1), 413–417.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201510990/full
▼Remote Tris(pentafluorophenyl)borane-Assisted Chiral Phosphoric Acid Catalysts for the Enantioselective Diels–Alder Reaction
Manabu Hatano, Hideyuki Ishihara, Yuta Goto, Kazuaki Ishihara*
Synlett, 2016, 27, 564-570.
DOI: 10.1055/s-0035-1560369
https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0035-1560369
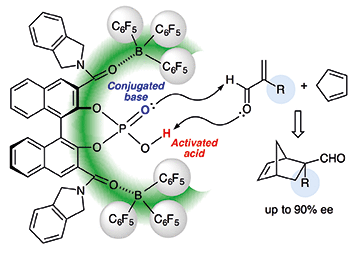
Tris(pentafluorophenyl)borane-assisted chiral supramolecular phosphoric acid catalysts were developed for the model Diels–Alder reaction of α-substituted acroleins with cyclopentadiene. Two remotely coordinated tris(pentafluorophenyl)boranes should help to increase the Brønsted acidity of the active center in the supramolecular catalyst and create effective bulkiness for the chiral cavity. The prepared supramolecular catalysts acted as not only conjugated Brønsted acid–Brønsted base catalysts but also bifunctional Lewis acid–Brønsted base catalysts with the addition of a central achiral Lewis acid source such as catecholborane.
▼Boronic Acid–DMAPO Cooperative Catalysis for Dehydrative Condensation between Carboxylic Acids and
Amines
Kazuaki Ishihara and Yanhui Lu
Chem. Sci. 2016, 7, 1276–1280. Accepted Manuscript (Received 04 Oct 2015, Accepted 02 Nov 2015)
DOI: 10.1039/C5SC03761A
http://pubs.rsc.org/en/content/articlelanding/2015/sc/c5sc03761a#!divAbstract

Arylboronic acid and 4-(N,N-dimethylamino)pyridine N-oxide (DMAPO) cooperatively catalyse the dehydrative condensation reaction between carboxylic acids and amines to give the corresponding amides under azeotropic reflux conditions. The cooperative use of them is much more effective than their individual use as catalyst, and chemoselectively promoted the amide condensation of (poly)conjugated carboxylic acids. The present method is readily practical and scalable, and has been applied to the synthesis of Sitagliptin and a drug candidate.














